

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CN[C@@H](C)C(=O)N[C@H]1[C@H](C)N(C(C)=O)c2cc(ccc2N(Cc2c(OC)ccc3ccccc23)C1=O)C#N
InChI Key: InChIKey=HIFDPWOYCPYURM-VFUKXHBFSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E3 ubiquitin-protein ligase XIAP [124-214,C202A,C213G] (Artificial Organism) | BDBM50441807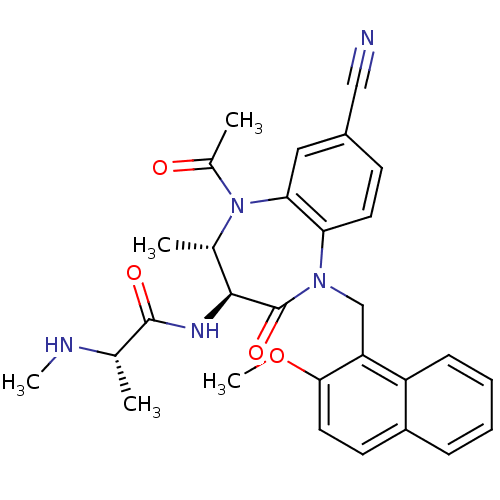 (CHEMBL2436225 | US9422331, 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.5 | 37 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description The peptide AVPIAQKSEK-(ε-biotin)-OH 1:2 TFA ("Peptide A") was identified as a substrate for the TR-FRET assay by screening the 6× Hist... | US Patent US9422331 (2016) BindingDB Entry DOI: 10.7270/Q2736PT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP [241-356] (Homo sapiens (Human)) | BDBM50441807 (CHEMBL2436225 | US9422331, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.32E+4 | n/a | n/a | n/a | n/a | 7.5 | 37 |
HOFFMANN-LA ROCHE INC. US Patent | Assay Description The peptide AVPIAQKSEK-(ε-biotin)-OH 1:2 TFA ("Peptide A") was identified as a substrate for the TR-FRET assay by screening the 6× Hist... | US Patent US9422331 (2016) BindingDB Entry DOI: 10.7270/Q2736PT4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441807 (CHEMBL2436225 | US9422331, 31) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR2 domain (124 to 240) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50441807 (CHEMBL2436225 | US9422331, 31) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 (unknown origin) | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| E3 ubiquitin-protein ligase XIAP (Homo sapiens (Human)) | BDBM50441807 (CHEMBL2436225 | US9422331, 31) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Displacement of AVPIAQKSEK (epsilon-biotin)-OH from 6xhistidine-thrombin-TEV-tagged XIAP BIR3 domain (241 to 356) (unknown origin) after 45 mins by T... | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50441807 (CHEMBL2436225 | US9422331, 31) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hoffmann-La Roche Inc. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | J Med Chem 56: 7788-803 (2013) Article DOI: 10.1021/jm400732v BindingDB Entry DOI: 10.7270/Q2M32X6V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||