

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: OP(O)(=O)C(Nc1ccc(Cl)c(Cl)c1)P(O)(O)=O
InChI Key: InChIKey=ADHSWFUIIRWNHD-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 1 (Homo sapiens (Human)) | BDBM50442512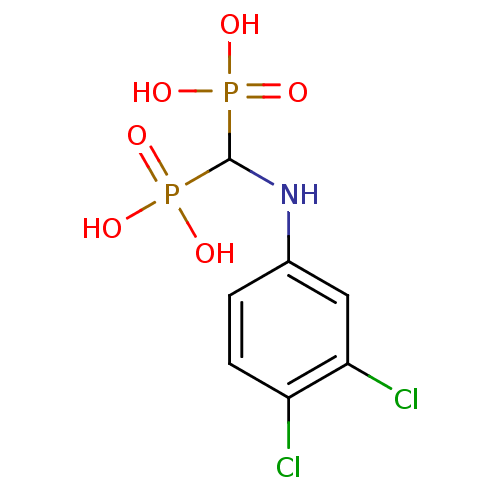 (CHEMBL2440433) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi 'Aldo Moro' di Bari Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 1 preincubated for 15 mins by stopped flow CO2 hydration method | Bioorg Med Chem Lett 24: 1941-3 (2014) Article DOI: 10.1016/j.bmcl.2014.03.001 BindingDB Entry DOI: 10.7270/Q2PV6MWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 2 (Homo sapiens (Human)) | BDBM50442512 (CHEMBL2440433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi 'Aldo Moro' di Bari Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 2 preincubated for 15 mins by stopped flow CO2 hydration method | Bioorg Med Chem Lett 24: 1941-3 (2014) Article DOI: 10.1016/j.bmcl.2014.03.001 BindingDB Entry DOI: 10.7270/Q2PV6MWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 9 (Homo sapiens (Human)) | BDBM50442512 (CHEMBL2440433) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi 'Aldo Moro' di Bari Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 9 preincubated for 15 mins by stopped flow CO2 hydration method | Bioorg Med Chem Lett 24: 1941-3 (2014) Article DOI: 10.1016/j.bmcl.2014.03.001 BindingDB Entry DOI: 10.7270/Q2PV6MWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 12 (Homo sapiens (Human)) | BDBM50442512 (CHEMBL2440433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi 'Aldo Moro' di Bari Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 12 preincubated for 15 mins by stopped flow CO2 hydration method | Bioorg Med Chem Lett 24: 1941-3 (2014) Article DOI: 10.1016/j.bmcl.2014.03.001 BindingDB Entry DOI: 10.7270/Q2PV6MWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyrroline-5-carboxylate reductase (Arabidopsis thaliana) | BDBM50442512 (CHEMBL2440433) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.68E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Inhibition of Arabidopsis thaliana P5C assessed as reduction in NADH oxidation incubated at 35 degC up to 10 min | J Agric Food Chem 56: 3193-9 (2008) Article DOI: 10.1021/jf800029t BindingDB Entry DOI: 10.7270/Q2N58Q73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50442512 (CHEMBL2440433) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi 'Aldo Moro' di Bari Curated by ChEMBL | Assay Description Inhibition of MMP14 catalytic domain (unknown origin) using Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 as substrate incubated for 30 mins prior to substrate... | Bioorg Med Chem 21: 6456-65 (2013) Article DOI: 10.1016/j.bmc.2013.08.054 BindingDB Entry DOI: 10.7270/Q2222W7K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamine synthetase, chloroplastic (Zea mays) | BDBM50442512 (CHEMBL2440433) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Inhibition of Zea mays cv. Jeff (maize) leaf glutamine synthetase using L-glutamate as substrate assessed as inorganic phosphate production after 20 ... | Pest Manag Sci 66: 51-8 (2010) Article DOI: 10.1002/ps.1830 BindingDB Entry DOI: 10.7270/Q2V40Z3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutamine synthetase, chloroplastic (Zea mays) | BDBM50442512 (CHEMBL2440433) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Inhibition of Zea mays cv. Jeff (maize) leaf glutamine synthetase using L-glutamate as substrate assessed as inorganic phosphate production after 20 ... | Pest Manag Sci 66: 51-8 (2010) Article DOI: 10.1002/ps.1830 BindingDB Entry DOI: 10.7270/Q2V40Z3P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pyrroline-5-carboxylate reductase (Arabidopsis thaliana) | BDBM50442512 (CHEMBL2440433) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.68E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Ferrara Curated by ChEMBL | Assay Description Inhibition of Arabidopsis thaliana P5C assessed as reduction in NADH oxidation incubated at 35 degC up to 10 min | J Agric Food Chem 56: 3193-9 (2008) Article DOI: 10.1021/jf800029t BindingDB Entry DOI: 10.7270/Q2N58Q73 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 14 (Homo sapiens (Human)) | BDBM50442512 (CHEMBL2440433) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.84E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi 'Aldo Moro' di Bari Curated by ChEMBL | Assay Description Inhibition of human recombinant carbonic anhydrase 14 preincubated for 15 mins by stopped flow CO2 hydration method | Bioorg Med Chem Lett 24: 1941-3 (2014) Article DOI: 10.1016/j.bmcl.2014.03.001 BindingDB Entry DOI: 10.7270/Q2PV6MWT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||