

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: N[C@@H]1C[C@H]1c1ccc(cc1)-c1cccc(O)c1
InChI Key: InChIKey=DSOJSZXQRJGBCW-LSDHHAIUSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50445349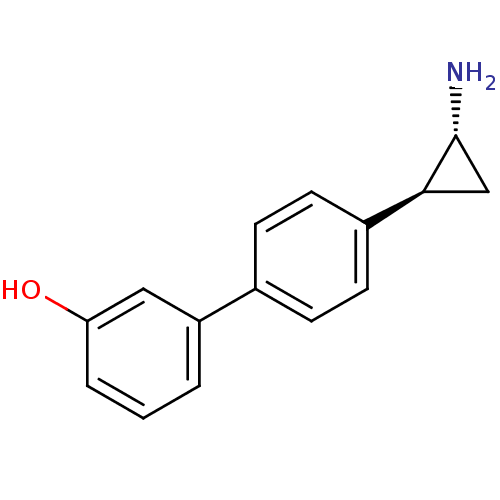 (CHEMBL3104261 | US9676701, 63 Enantiomers of 4R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | US Patent | 50 | -9.95 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
ORYZON GENOMICS, S.A. US Patent | Assay Description Briefly, a fixed amount of LSD1 was incubated on ice for 15 minutes, in the absence and/or in the presence of various concentrations of inhibitor (e.... | US Patent US9676701 (2017) BindingDB Entry DOI: 10.7270/Q27P8WJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50445349 (CHEMBL3104261 | US9676701, 63 Enantiomers of 4R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | US Patent | 50 | -9.95 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
ORYZON GENOMICS, S.A. US Patent | Assay Description Briefly, a fixed amount of LSD1 was incubated on ice for 15 minutes, in the absence and/or in the presence of various concentrations of inhibitor (e.... | US Patent US9676701 (2017) BindingDB Entry DOI: 10.7270/Q27P8WJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50445349 (CHEMBL3104261 | US9676701, 63 Enantiomers of 4R...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
ORYZON GENOMICS, S.A. US Patent | Assay Description Assays were conducted in 96-well black plates with clear bottom (Corning) in a final volume of 100 μL. The assay buffer was 100 mM HEPES, pH 7.5... | US Patent US9676701 (2017) BindingDB Entry DOI: 10.7270/Q27P8WJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM50445349 (CHEMBL3104261 | US9676701, 63 Enantiomers of 4R...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
ORYZON GENOMICS, S.A. US Patent | Assay Description Assays were conducted in 96-well black plates with clear bottom (Corning) in a final volume of 100 μL. The assay buffer was 100 mM HEPES, pH 7.5... | US Patent US9676701 (2017) BindingDB Entry DOI: 10.7270/Q27P8WJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50445349 (CHEMBL3104261 | US9676701, 63 Enantiomers of 4R...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
ORYZON GENOMICS, S.A. US Patent | Assay Description Assays were conducted in 96-well black plates with clear bottom (Corning) in a final volume of 100 μL. The assay buffer was 100 mM HEPES, pH 7.5... | US Patent US9676701 (2017) BindingDB Entry DOI: 10.7270/Q27P8WJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM50445349 (CHEMBL3104261 | US9676701, 63 Enantiomers of 4R...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | US Patent | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
ORYZON GENOMICS, S.A. US Patent | Assay Description Assays were conducted in 96-well black plates with clear bottom (Corning) in a final volume of 100 μL. The assay buffer was 100 mM HEPES, pH 7.5... | US Patent US9676701 (2017) BindingDB Entry DOI: 10.7270/Q27P8WJN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50445349 (CHEMBL3104261 | US9676701, 63 Enantiomers of 4R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Utah Curated by ChEMBL | Assay Description Inhibition of human recombinant LSD1 using dimethylated H3K4 peptide as substrate after 1 hr | J Med Chem 56: 9496-508 (2014) Article DOI: 10.1021/jm400870h BindingDB Entry DOI: 10.7270/Q2Z60QJ7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lysine-specific histone demethylase 1A (Homo sapiens (Human)) | BDBM50445349 (CHEMBL3104261 | US9676701, 63 Enantiomers of 4R...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | n/a | n/a | 4.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||