

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: ONC(=O)[C@@H]1[C@@H]([C@H]1c1ccc(cc1)-c1cnc(o1)C1CC1)c1ccccc1
InChI Key: InChIKey=VFVVXZLMBWETAN-VAMGGRTRSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50446470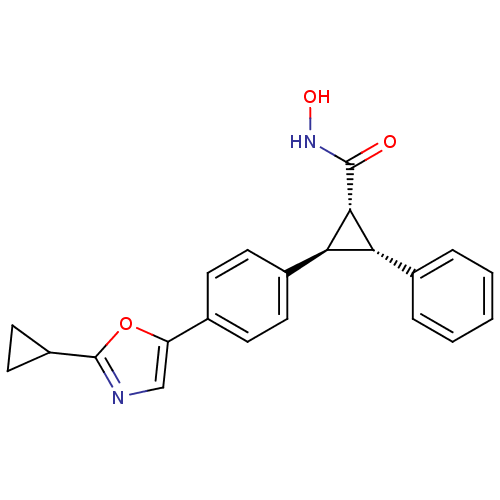 (CHEMBL3109985 | US9765054, Compound 46) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BioFocus Curated by ChEMBL | Assay Description Inhibition of human CYP2D6 | J Med Chem 56: 9934-54 (2013) Article DOI: 10.1021/jm4011884 BindingDB Entry DOI: 10.7270/Q2DZ09RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50446470 (CHEMBL3109985 | US9765054, Compound 46) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US9765054 (2017) BindingDB Entry DOI: 10.7270/Q2XW4MZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50446470 (CHEMBL3109985 | US9765054, Compound 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
BioFocus Curated by ChEMBL | Assay Description Inhibition of human CYP2C9 | J Med Chem 56: 9934-54 (2013) Article DOI: 10.1021/jm4011884 BindingDB Entry DOI: 10.7270/Q2DZ09RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50446470 (CHEMBL3109985 | US9765054, Compound 46) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
BioFocus Curated by ChEMBL | Assay Description Inhibition of human CYP2C19 | J Med Chem 56: 9934-54 (2013) Article DOI: 10.1021/jm4011884 BindingDB Entry DOI: 10.7270/Q2DZ09RK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||