

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50447178 CHEMBL1596118::US10752581, Compound 1
SMILES: COc1cccc(CNc2ccc(cc2)S(=O)(=O)Nc2nccs2)c1O
InChI Key: InChIKey=CENSVXZQMJBVHY-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50447178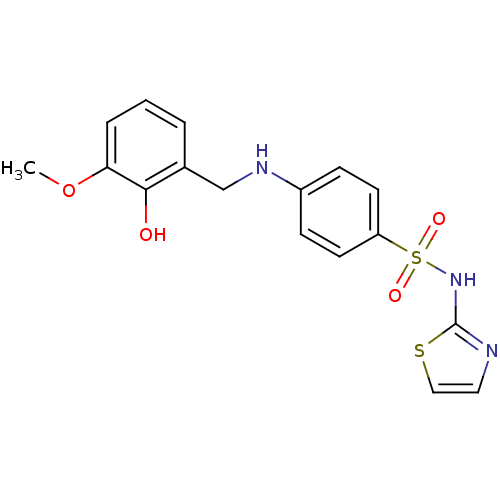 (CHEMBL1596118 | US10752581, Compound 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of human 5-LOX using arachidonic acid as substrate by UV-vis spectrophotometric analysis | J Med Chem 57: 495-506 (2014) Article DOI: 10.1021/jm4016476 BindingDB Entry DOI: 10.7270/Q27082XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 15-Lipoxygenase-2 (15-LOX-2) (Homo sapiens (Human)) | BDBM50447178 (CHEMBL1596118 | US10752581, Compound 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human epithelial 15-LOX2 using arachidonic acid as substrate by UV-vis spectrophotometric analysis | J Med Chem 57: 495-506 (2014) Article DOI: 10.1021/jm4016476 BindingDB Entry DOI: 10.7270/Q27082XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 12-lipoxygenase (Homo sapiens (Human)) | BDBM50447178 (CHEMBL1596118 | US10752581, Compound 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Eastern Virginia Medical School; The Regents of the University of California Santa; The United States of America Department of Health; Thomas Jefferson University US Patent | Assay Description UV-vis cuvette-based assay. | US Patent US10752581 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 12-Lipoxygenase (12-LOX) (Homo sapiens (Human)) | BDBM50447178 (CHEMBL1596118 | US10752581, Compound 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human platelet 12-LOX using arachidonic acid as substrate by UV-vis spectrophotometric analysis | J Med Chem 57: 495-506 (2014) Article DOI: 10.1021/jm4016476 BindingDB Entry DOI: 10.7270/Q27082XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 15-lipoxygenase (Homo sapiens (Human)) | BDBM50447178 (CHEMBL1596118 | US10752581, Compound 1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institutes of Health Curated by ChEMBL | Assay Description Inhibition of N-terminal His6-tagged human reticulocyte 15-LOX1 using arachidonic acid as substrate by UV-vis spectrophotometric analysis | J Med Chem 57: 495-506 (2014) Article DOI: 10.1021/jm4016476 BindingDB Entry DOI: 10.7270/Q27082XR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||