

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50449963 CHEMBL4176701
SMILES: CN(C[C@H]1Cc2c(CN1)cccc2N1CCNCC1)[C@H]1CCCc2cc(C)cnc12
InChI Key: InChIKey=RSENRXMUUIGFKE-QPPBQGQZSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Muscarinic acetylcholine receptor (Homo sapiens (Human)) | BDBM50449963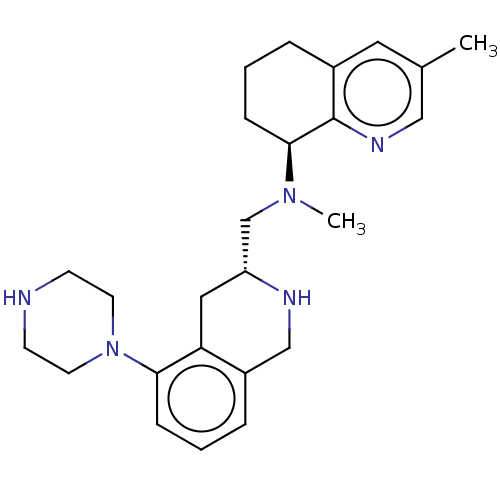 (CHEMBL4176701) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium release preincubated for 25 mins followe... | J Med Chem 61: 7168-7188 (2018) Article DOI: 10.1021/acs.jmedchem.8b00450 BindingDB Entry DOI: 10.7270/Q2GH9MJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50449963 (CHEMBL4176701) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP2D6 expressed in insect cells microsomes using AMMC as substrate preincubated for 30 mins followed by NADP additio... | J Med Chem 61: 7168-7188 (2018) Article DOI: 10.1021/acs.jmedchem.8b00450 BindingDB Entry DOI: 10.7270/Q2GH9MJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50449963 (CHEMBL4176701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Agonist activity at CXCR4 in human CCRF-CEM cells assessed as induction of calcium release incubated for 25 mins by calcium flux assay | J Med Chem 61: 7168-7188 (2018) Article DOI: 10.1021/acs.jmedchem.8b00450 BindingDB Entry DOI: 10.7270/Q2GH9MJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 4 (Homo sapiens (Human)) | BDBM50449963 (CHEMBL4176701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Antagonist activity at CXCR4 in human CCRF-CEM cells assessed as inhibition of SDF-1alpha-induced calcium release preincubated for 25 mins followed b... | J Med Chem 61: 7168-7188 (2018) Article DOI: 10.1021/acs.jmedchem.8b00450 BindingDB Entry DOI: 10.7270/Q2GH9MJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50449963 (CHEMBL4176701) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Curated by ChEMBL | Assay Description Inhibition of human CYP3A4 | J Med Chem 61: 7168-7188 (2018) Article DOI: 10.1021/acs.jmedchem.8b00450 BindingDB Entry DOI: 10.7270/Q2GH9MJ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||