

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50450953 CHEMBL4209248
SMILES: CCc1nn2c(C)cc(C)nc2c1Cc1ccc(\C=C\CN2CCN(CC2)C(C)C)cc1
InChI Key: InChIKey=CSQZLIGLFJJGNT-BQYQJAHWSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50450953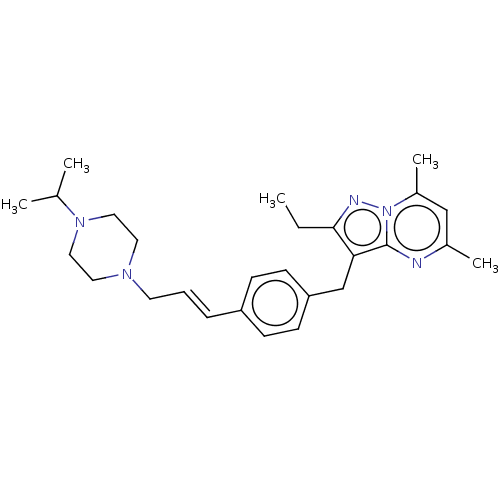 (CHEMBL4209248) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]-R(-)-alpha-Methyl[imidazole-2.5(n)]histamine from human recombinant histamine H3 receptor expressed in CHOK1 cell membranes afte... | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50450953 (CHEMBL4209248) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Displacement of [3H]dofetilide from recombinant human ERG expressed in HEK293 cell membranes after 90 mins by liquid scintillation counting method | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| G-protein coupled receptor 4 (GPR4) (Homo sapiens (Human)) | BDBM50450953 (CHEMBL4209248) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research Curated by ChEMBL | Assay Description Antagonist activity at human GPR4 expressed in human HeLa cells assessed as inhibition of IBMX-induced cAMP accumulation after 15 mins by HTRF assay | J Med Chem 60: 3672-3683 (2017) Article DOI: 10.1021/acs.jmedchem.6b01703 BindingDB Entry DOI: 10.7270/Q28P6349 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||