

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50466892 CHEMBL4283871
SMILES: O=C(Nc1ccc2[nH]cc(C3CCN(CC3)C(=O)C3CCCC3)c2c1)c1cccc(c1)C#N
InChI Key: InChIKey=HDYDGYGIQGBWPQ-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM50466892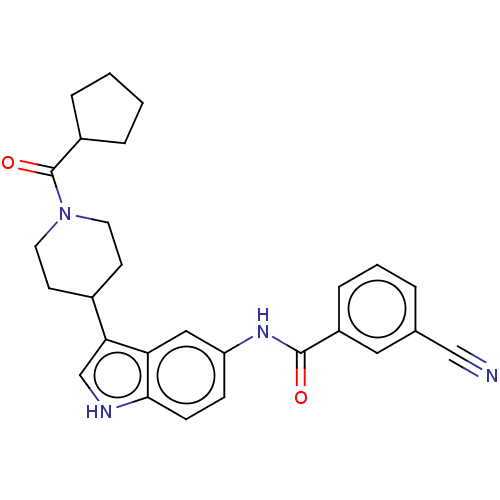 (CHEMBL4283871) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Displacement of [3H]-25-hydroxycholesterol from human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cell... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-alpha (Homo sapiens (Human)) | BDBM50466892 (CHEMBL4283871) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at RORA (unknown origin) assessed as inhibition of biotinylated co-activator SRC1-2 peptide recruitment after 3 hrs by TR-FR... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-beta (Homo sapiens (Human)) | BDBM50466892 (CHEMBL4283871) | PDB MMDB Reactome pathway UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4-fused RORB LBD (unknown origin) expressed in mouse neuro2A cells assessed as inhibition of transcriptional activity ... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-beta (Homo sapiens (Human)) | BDBM50466892 (CHEMBL4283871) | PDB MMDB Reactome pathway UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at RORB (unknown origin) assessed as inhibition of biotinylated co-activator SRC1-2 peptide recruitment after 3 hrs by TR-FR... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM50466892 (CHEMBL4283871) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at RORC2 in human Th17 cells assessed as inhibition of IL17A production after 6 days by sandwich ELISA | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM50466892 (CHEMBL4283871) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4-fused RORC2 LBD (unknown origin) expressed in mouse neuro2A cells assessed as inhibition of transcriptional activity... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-alpha (Homo sapiens (Human)) | BDBM50466892 (CHEMBL4283871) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at GAL4-fused RORA LBD (unknown origin) expressed in mouse neuro2A cells assessed as inhibition of transcriptional activity ... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nuclear receptor ROR-gamma (RORC) (Homo sapiens (Human)) | BDBM50466892 (CHEMBL4283871) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Karo Bio AB (now Karo Pharma AB) Curated by ChEMBL | Assay Description Inverse agonist activity at human N-terminal 6His-tagged-RORC2 LBD (259 to 483 residues) expressed in Escherichia coli (DE3) cells assessed as inhibi... | J Med Chem 61: 10415-10439 (2018) Article DOI: 10.1021/acs.jmedchem.8b00392 BindingDB Entry DOI: 10.7270/Q27P922T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||