 Found 20 hits for monomerid = 50469457
Found 20 hits for monomerid = 50469457 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50469457
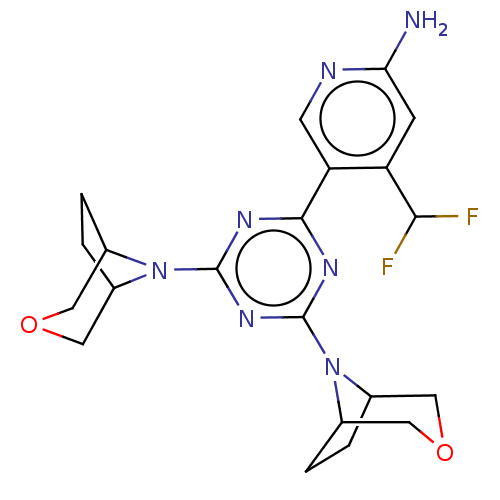
(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant human GST-tagged mTOR catalytic domain (1360 to 2549 residues) expressed in baculovirus after 1 hr by TR-FRET displac... |
J Med Chem 61: 10084-10105 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01262
BindingDB Entry DOI: 10.7270/Q2X92F07 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of alexa fluor 647-labeled kinase tracer 314 binding to recombinant human N-terminal GST-fused mTOR (1360 to 2549 residues) expressed in b... |
J Med Chem 62: 8609-8630 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00972 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to recombinant N-terminal His6-tagged P110alpha catalytic domain (unknown origin) after 1 hr by TR-FRET displacement assay |
J Med Chem 61: 10084-10105 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01262
BindingDB Entry DOI: 10.7270/Q2X92F07 |
More data for this
Ligand-Target Pair | |
p110α/p85α
(Homo sapiens (Human)) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of alexa fluor 647-labeled kinase tracer 314 binding to recombinant human full-length N-terminal His6-tagged p110alpha/p85alpha expressed ... |
J Med Chem 62: 8609-8630 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00972 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase beta
(Homo sapiens (Human)) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human PI4Kbeta (1 to 828 residues) expressed in mammalian expression system by KINOMEscan assay |
J Med Chem 61: 10084-10105 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01262
BindingDB Entry DOI: 10.7270/Q2X92F07 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kalpha (108 to 1068 residues) expressed in mammalian expression system by KINOMEscan assay |
J Med Chem 61: 10084-10105 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01262
BindingDB Entry DOI: 10.7270/Q2X92F07 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human VPS34 (282 to 879 residues) expressed in mammalian expression system by KINOMEscan assay |
J Med Chem 61: 10084-10105 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01262
BindingDB Entry DOI: 10.7270/Q2X92F07 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human wild-type partial length PI3Kbeta (P118 to S1070 residues) expressed in mammalian expression system by kinome scan assay |
J Med Chem 62: 8609-8630 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00972 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex subunit LST8
(Homo sapiens) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTORC1 in human A2058 cells assessed as reduction in ribosomal protein S6 phosphorylation at Ser235/236 residues incubated for 1 hr by ... |
J Med Chem 62: 8609-8630 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00972 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human wild-type partial length PI3Kdelta (R108 to Q1044 residues) expressed in mammalian expression system by kinome scan assay |
J Med Chem 62: 8609-8630 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00972 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human wild-type partial length PI3Kalpha (R108 to N1068 residues) expressed in mammalian expression system by kinome scan assay |
J Med Chem 62: 8609-8630 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00972 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human wild-type partial length PI3Kgamma (S144 to A1102 residues) expressed in mammalian expression system by kinome scan assay |
J Med Chem 62: 8609-8630 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00972 |
More data for this
Ligand-Target Pair | |
Target of rapamycin complex 2 subunit MAPKAP1
(Homo sapiens) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of mTORC2 in human A2058 cells assessed as reduction in PKB phosphorylation at S473 residues incubated for 1 hr by Western blot analysis |
J Med Chem 62: 8609-8630 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00972 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to wild-type human partial length mTOR (L1382 to W2549 residues) expressed in mammalian expression system by kinome scan assay |
J Med Chem 62: 8609-8630 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00972 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase, PI4K
(Homo sapiens (Human)) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to wild-type human full-length PI4Kbeta (M1 to M828 residues) expressed in mammalian expression system by kinome scan assay |
J Med Chem 62: 8609-8630 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00972 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kgamma (144 to 1102 residues) expressed in mammalian expression system by KINOMEscan assay |
J Med Chem 61: 10084-10105 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01262
BindingDB Entry DOI: 10.7270/Q2X92F07 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kdelta (108 to 1044 residues) expressed in mammalian expression system by KINOMEscan assay |
J Med Chem 61: 10084-10105 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01262
BindingDB Entry DOI: 10.7270/Q2X92F07 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.75E+3 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Binding affinity to human wild-type partial length VPS34 (S282 to H879 residues) expressed in mammalian expression system by kinome scan assay |
J Med Chem 62: 8609-8630 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00972 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human mTOR (1382 to 2549 residues) expressed in mammalian expression system by KINOMEscan assay |
J Med Chem 61: 10084-10105 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01262
BindingDB Entry DOI: 10.7270/Q2X92F07 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50469457

(CHEMBL4286698)Show SMILES Nc1cc(C(F)F)c(cn1)-c1nc(nc(n1)N1C2CCC1COC2)N1C2CCC1COC2 Show InChI InChI=1S/C21H25F2N7O2/c22-18(23)15-5-17(24)25-6-16(15)19-26-20(29-11-1-2-12(29)8-31-7-11)28-21(27-19)30-13-3-4-14(30)10-32-9-13/h5-6,11-14,18H,1-4,7-10H2,(H2,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a |
University of Basel
Curated by ChEMBL
| Assay Description
Inhibition of human PI3Kbeta (118 to 1070 residues) expressed in mammalian expression system by KINOMEscan assay |
J Med Chem 61: 10084-10105 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01262
BindingDB Entry DOI: 10.7270/Q2X92F07 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data