

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50469861 CHEMBL438829
SMILES: [#6]-[#6](-[#6])-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@H](-[#6]-[#6]-[#6]-[#7]-[#6](-[#7])=O)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(-[#8])cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-[#8])-[#7]-[#6](=O)-[#6@H](-[#6]-c1cccnc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc(Cl)cc1)-[#7]-[#6](=O)-[#6@H](-[#6]-c1ccc2ccccc2c1)-[#7]-[#6](-[#6])=O)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-1-[#6]-[#6]-[#6]-[#6@@H]-1-[#6](=O)-[#7]-[#6@@H](-[#6])-[#6](-[#7])=O
InChI Key: InChIKey=SBNPWPIBESPSIF-JIFAMXAPSA-N
Data: 1 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gonadotropin-releasing hormone receptor (Rattus norvegicus) | BDBM50469861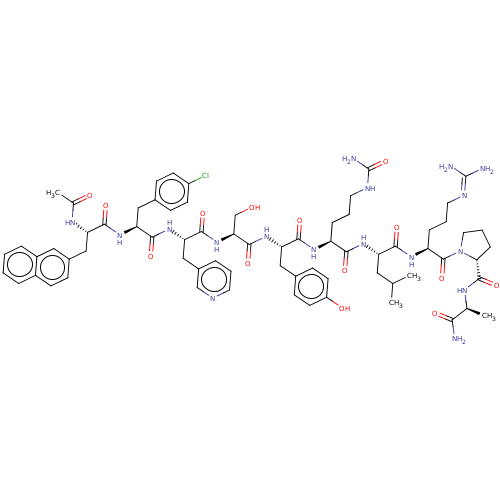 (CHEMBL438829) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 0.117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description The negative logarithm of the concentration of antagonist that inhibits 50% of the binding of 125 I-labeled leuprolide to the rat pituitary LHRH rece... | J Med Chem 36: 928-33 (1993) Article DOI: 10.1021/jm00059a020 BindingDB Entry DOI: 10.7270/Q2Z03BW7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||