

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC1=C([C@@H](Oc2cc(OC(=O)C(C)(C)C)ccc12)c1ccc(OCCN2CCCCC2)cc1)c1ccc(OC(=O)C(C)(C)C)cc1
InChI Key: InChIKey=OEKMGABCSLYWOP-DHUJRADRSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471254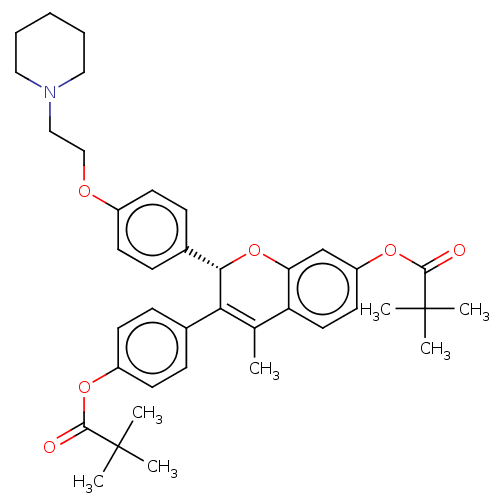 (CHEMBL308234) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent binding affinity against estradiol-stimulated T-47D cell proliferation | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471254 (CHEMBL308234) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Stimulation of alkaline phosphatase activity in human endometrial Ishikawa cells with 1 nM E2 estradiol | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471254 (CHEMBL308234) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Inhibition of estradiol binding to estrogen receptor in Human Breast cancer cytosol (3.3% ethanol) | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471254 (CHEMBL308234) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Apparent inhibition constant for estrogen receptor in Human uterine cytosol in 3.3%ethanol | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471254 (CHEMBL308234) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Stimulation of alkaline phosphatase activity in human endometrial Ishikawa cells with 1 nM E2 estradiol | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor beta (Homo sapiens (Human)) | BDBM50471254 (CHEMBL308234) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Le Centre Hospitalier Universitaire de Qu£bec Curated by ChEMBL | Assay Description Inhibition of estradiol-stimulated T-47D cell proliferation. | J Med Chem 40: 2117-22 (1997) Article DOI: 10.1021/jm970095o BindingDB Entry DOI: 10.7270/Q2474DKC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||