

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50475690 4,4''-Diallylcaracurinium V Dibromide::DIALLYLCARACURIE V::Diallylcaracurie V
SMILES: [Br-].[Br-].[H][C@]12C[C@@]3([H])C4=CCO[C@@]5([H])N6c7ccccc7[C@@]78CC[N+]9(CC=C)CC%10=CCO[C@@]([H])(N%11c%12ccccc%12[C@@]1(CC[N+]2(CC=C)C4)[C@]%11([H])[C@@]35[H])[C@@]([H])([C@]67[H])[C@@]%10([H])C[C@@]89[H]
InChI Key: InChIKey=IULOHWLFKDQAPI-QHKRVHTNSA-L
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholine receptor protein alpha/beta/delta/gamma chain (Torpedo californica) | BDBM50475690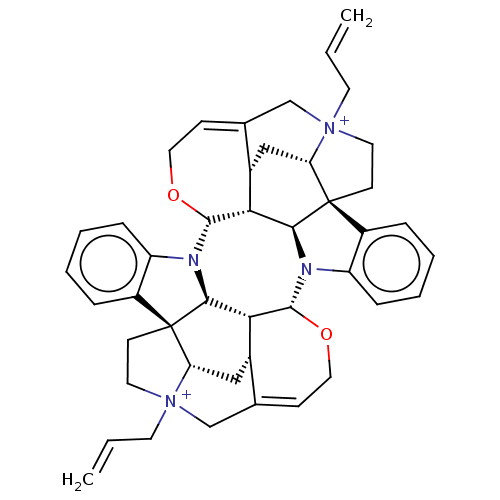 (4,4''-Diallylcaracurinium V Dibromide | DIALLYLCAR...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Displacement of (+/-)-[3H]epibatidine from muscle type nAChR of Torpedo californica in hepes buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 and M5 (Homo sapiens (Human)) | BDBM50475690 (4,4''-Diallylcaracurinium V Dibromide | DIALLYLCAR...) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 11 | n/a | n/a | n/a | n/a |
University of W£rzburg Curated by ChEMBL | Assay Description Inhibition of [3H]NMS dissociation from muscarinic M2 receptor in Na,K,Pi buffer | Bioorg Med Chem Lett 16: 1481-5 (2006) Article DOI: 10.1016/j.bmcl.2005.12.030 BindingDB Entry DOI: 10.7270/Q2D50QQ5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||