 Found 7 hits for monomerid = 50483329
Found 7 hits for monomerid = 50483329 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Reverse Transcriptase
(Human immunodeficiency virus 1) | BDBM50483329
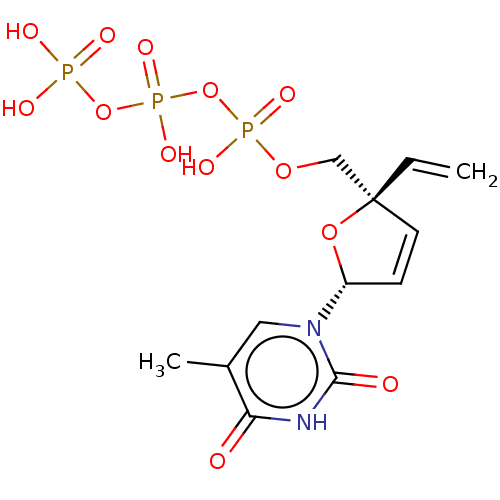
(CHEMBL1650290)Show SMILES Cc1cn([C@@H]2O[C@@](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)(C=C)C=C2)c(=O)[nH]c1=O |r,c:23| Show InChI InChI=1S/C12H17N2O13P3/c1-3-12(7-24-29(20,21)27-30(22,23)26-28(17,18)19)5-4-9(25-12)14-6-8(2)10(15)13-11(14)16/h3-6,9H,1,7H2,2H3,(H,20,21)(H,22,23)(H,13,15,16)(H2,17,18,19)/t9-,12+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 Reverse transcriptase P119S/T165A mutant |
Antimicrob Agents Chemother 53: 4640-6 (2009)
Article DOI: 10.1128/aac.00686-09
BindingDB Entry DOI: 10.7270/Q2MC92VF |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483329

(CHEMBL1650290)Show SMILES Cc1cn([C@@H]2O[C@@](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)(C=C)C=C2)c(=O)[nH]c1=O |r,c:23| Show InChI InChI=1S/C12H17N2O13P3/c1-3-12(7-24-29(20,21)27-30(22,23)26-28(17,18)19)5-4-9(25-12)14-6-8(2)10(15)13-11(14)16/h3-6,9H,1,7H2,2H3,(H,20,21)(H,22,23)(H,13,15,16)(H2,17,18,19)/t9-,12+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of wild-type HIV1 Reverse transcriptase |
Antimicrob Agents Chemother 53: 4640-6 (2009)
Article DOI: 10.1128/aac.00686-09
BindingDB Entry DOI: 10.7270/Q2MC92VF |
More data for this
Ligand-Target Pair | |
Reverse transcriptase
(Human immunodeficiency virus 1) | BDBM50483329

(CHEMBL1650290)Show SMILES Cc1cn([C@@H]2O[C@@](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)(C=C)C=C2)c(=O)[nH]c1=O |r,c:23| Show InChI InChI=1S/C12H17N2O13P3/c1-3-12(7-24-29(20,21)27-30(22,23)26-28(17,18)19)5-4-9(25-12)14-6-8(2)10(15)13-11(14)16/h3-6,9H,1,7H2,2H3,(H,20,21)(H,22,23)(H,13,15,16)(H2,17,18,19)/t9-,12+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 Reverse transcriptase T165A mutant |
Antimicrob Agents Chemother 53: 4640-6 (2009)
Article DOI: 10.1128/aac.00686-09
BindingDB Entry DOI: 10.7270/Q2MC92VF |
More data for this
Ligand-Target Pair | |
Reverse Transcriptase
(Human immunodeficiency virus 1) | BDBM50483329

(CHEMBL1650290)Show SMILES Cc1cn([C@@H]2O[C@@](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)(C=C)C=C2)c(=O)[nH]c1=O |r,c:23| Show InChI InChI=1S/C12H17N2O13P3/c1-3-12(7-24-29(20,21)27-30(22,23)26-28(17,18)19)5-4-9(25-12)14-6-8(2)10(15)13-11(14)16/h3-6,9H,1,7H2,2H3,(H,20,21)(H,22,23)(H,13,15,16)(H2,17,18,19)/t9-,12+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 Reverse transcriptase P119S mutant |
Antimicrob Agents Chemother 53: 4640-6 (2009)
Article DOI: 10.1128/aac.00686-09
BindingDB Entry DOI: 10.7270/Q2MC92VF |
More data for this
Ligand-Target Pair | |
Reverse Transcriptase
(Human immunodeficiency virus 1) | BDBM50483329

(CHEMBL1650290)Show SMILES Cc1cn([C@@H]2O[C@@](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)(C=C)C=C2)c(=O)[nH]c1=O |r,c:23| Show InChI InChI=1S/C12H17N2O13P3/c1-3-12(7-24-29(20,21)27-30(22,23)26-28(17,18)19)5-4-9(25-12)14-6-8(2)10(15)13-11(14)16/h3-6,9H,1,7H2,2H3,(H,20,21)(H,22,23)(H,13,15,16)(H2,17,18,19)/t9-,12+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 Reverse transcriptase M184V mutant |
Antimicrob Agents Chemother 53: 4640-6 (2009)
Article DOI: 10.1128/aac.00686-09
BindingDB Entry DOI: 10.7270/Q2MC92VF |
More data for this
Ligand-Target Pair | |
Reverse Transcriptase
(Human immunodeficiency virus 1) | BDBM50483329

(CHEMBL1650290)Show SMILES Cc1cn([C@@H]2O[C@@](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)(C=C)C=C2)c(=O)[nH]c1=O |r,c:23| Show InChI InChI=1S/C12H17N2O13P3/c1-3-12(7-24-29(20,21)27-30(22,23)26-28(17,18)19)5-4-9(25-12)14-6-8(2)10(15)13-11(14)16/h3-6,9H,1,7H2,2H3,(H,20,21)(H,22,23)(H,13,15,16)(H2,17,18,19)/t9-,12+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 Reverse transcriptase P119S/M184V mutant |
Antimicrob Agents Chemother 53: 4640-6 (2009)
Article DOI: 10.1128/aac.00686-09
BindingDB Entry DOI: 10.7270/Q2MC92VF |
More data for this
Ligand-Target Pair | |
Reverse Transcriptase
(Human immunodeficiency virus 1) | BDBM50483329

(CHEMBL1650290)Show SMILES Cc1cn([C@@H]2O[C@@](COP(O)(=O)OP(O)(=O)OP(O)(O)=O)(C=C)C=C2)c(=O)[nH]c1=O |r,c:23| Show InChI InChI=1S/C12H17N2O13P3/c1-3-12(7-24-29(20,21)27-30(22,23)26-28(17,18)19)5-4-9(25-12)14-6-8(2)10(15)13-11(14)16/h3-6,9H,1,7H2,2H3,(H,20,21)(H,22,23)(H,13,15,16)(H2,17,18,19)/t9-,12+/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yale University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 Reverse transcriptase P119S/T165A/M184V mutant |
Antimicrob Agents Chemother 53: 4640-6 (2009)
Article DOI: 10.1128/aac.00686-09
BindingDB Entry DOI: 10.7270/Q2MC92VF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data