

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50486103 CHEMBL2203874
SMILES: [H][C@@]12C[C@H](N(C1)C(=O)[C@@H](NC(=O)O[C@]1([H])CCC[C@@]1([H])C\C=C\c1cc3c(O2)cc(OCC)nc3cc1OC)C1CCCC1)C(=O)N[C@@]1(C[C@H]1C=C)C(=O)NS(=O)(=O)C1CC1
InChI Key: InChIKey=RWMPLRLPTAINLR-OFPQYJPJSA-N
Data: 2 KI
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepacivirus C) | BDBM50486103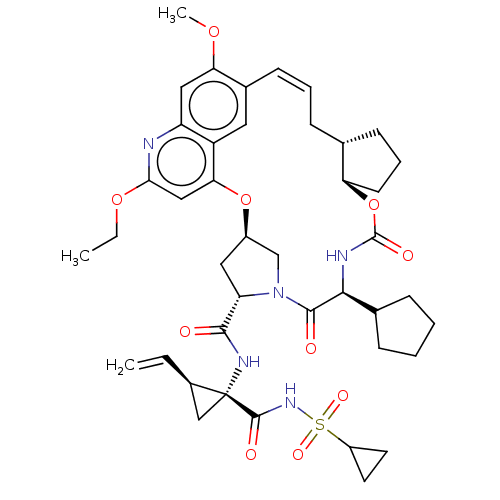 (CHEMBL2203874) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156T mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepacivirus C) | BDBM50486103 (CHEMBL2203874) | PDB Reactome pathway KEGG UniProtKB/TrEMBL DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of HCV genotype 1b BK NS3/4A protease A156V mutant expressed in Escherichia coli incubated for 30 mins by time-resolved fluorescence assay | Bioorg Med Chem Lett 22: 7207-13 (2012) Article DOI: 10.1016/j.bmcl.2012.09.061 BindingDB Entry DOI: 10.7270/Q28D0041 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||