 Found 5 hits for monomerid = 50502387
Found 5 hits for monomerid = 50502387 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Nuclear receptor ROR-gamma (RORC)
(Homo sapiens (Human)) | BDBM50502387
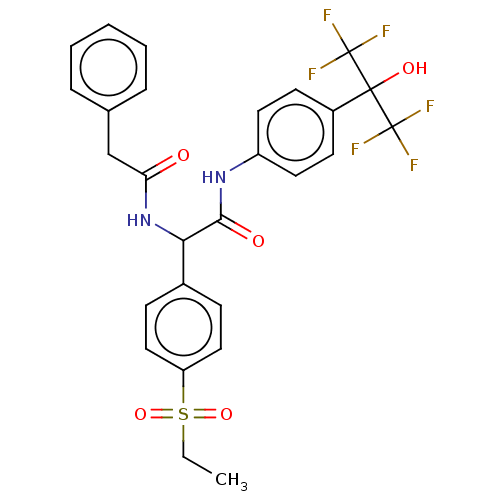
(CHEMBL4458320)Show SMILES CCS(=O)(=O)c1ccc(cc1)C(NC(=O)Cc1ccccc1)C(=O)Nc1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H24F6N2O5S/c1-2-41(39,40)21-14-8-18(9-15-21)23(35-22(36)16-17-6-4-3-5-7-17)24(37)34-20-12-10-19(11-13-20)25(38,26(28,29)30)27(31,32)33/h3-15,23,38H,2,16H2,1H3,(H,34,37)(H,35,36) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of tritiated 2-(4-(ethylsulfonyl)phenyl)-N-(4-(2-(methoxymethyl)phenyl)thiophen-2-yl)acetamide from human N-(HN)6-GST-TCS-RORC LBD (258 ... |
ACS Med Chem Lett 10: 972-977 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00158 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-alpha
(Homo sapiens (Human)) | BDBM50502387

(CHEMBL4458320)Show SMILES CCS(=O)(=O)c1ccc(cc1)C(NC(=O)Cc1ccccc1)C(=O)Nc1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H24F6N2O5S/c1-2-41(39,40)21-14-8-18(9-15-21)23(35-22(36)16-17-6-4-3-5-7-17)24(37)34-20-12-10-19(11-13-20)25(38,26(28,29)30)27(31,32)33/h3-15,23,38H,2,16H2,1H3,(H,34,37)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Agonist activity at human His6-tcs-RORalpha LBD assessed as PGC1alpha peptide (130 to 154 residues) recruitment incubated for 1 hr by FRET assay |
ACS Med Chem Lett 10: 972-977 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00158 |
More data for this
Ligand-Target Pair | |
Nuclear receptor ROR-gamma (RORC)
(Homo sapiens (Human)) | BDBM50502387

(CHEMBL4458320)Show SMILES CCS(=O)(=O)c1ccc(cc1)C(NC(=O)Cc1ccccc1)C(=O)Nc1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H24F6N2O5S/c1-2-41(39,40)21-14-8-18(9-15-21)23(35-22(36)16-17-6-4-3-5-7-17)24(37)34-20-12-10-19(11-13-20)25(38,26(28,29)30)27(31,32)33/h3-15,23,38H,2,16H2,1H3,(H,34,37)(H,35,36) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inverse agonist activity at human biotinylated HN-Avi-MBPS-TCS-RORC2 (258 to 518 residues) assessed as recruitment of SRC-1-derived coactivator measu... |
ACS Med Chem Lett 10: 972-977 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00158 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma
(Mus musculus) | BDBM50502387

(CHEMBL4458320)Show SMILES CCS(=O)(=O)c1ccc(cc1)C(NC(=O)Cc1ccccc1)C(=O)Nc1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H24F6N2O5S/c1-2-41(39,40)21-14-8-18(9-15-21)23(35-22(36)16-17-6-4-3-5-7-17)24(37)34-20-12-10-19(11-13-20)25(38,26(28,29)30)27(31,32)33/h3-15,23,38H,2,16H2,1H3,(H,34,37)(H,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of tritiated 2-(4-(ethylsulfonyl)phenyl)-N-(4-(2-(methoxymethyl)phenyl)thiophen-2-yl)acetamide from mouse HN-AVI-GST-TCS-RORC (256 to 51... |
ACS Med Chem Lett 10: 972-977 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00158 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Nuclear receptor ROR-gamma (RORC)
(Homo sapiens (Human)) | BDBM50502387

(CHEMBL4458320)Show SMILES CCS(=O)(=O)c1ccc(cc1)C(NC(=O)Cc1ccccc1)C(=O)Nc1ccc(cc1)C(O)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H24F6N2O5S/c1-2-41(39,40)21-14-8-18(9-15-21)23(35-22(36)16-17-6-4-3-5-7-17)24(37)34-20-12-10-19(11-13-20)25(38,26(28,29)30)27(31,32)33/h3-15,23,38H,2,16H2,1H3,(H,34,37)(H,35,36) | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inverse agonist activity at RORC2 in human Th17 cells assessed as inhibition of IL-17A secretion incubated for 4 days by HTRF assay |
ACS Med Chem Lett 10: 972-977 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00158 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data