

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50503240 CHEMBL4445651
SMILES: Nc1nc2[nH]c(CCCc3csc(C(=O)N[C@@H](CCC(O)=O)C(O)=O)c3F)cc2c(=O)[nH]1
InChI Key: InChIKey=WMTFSFGBNBUALY-NSHDSACASA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proton-coupled folate transporter (Homo sapiens (Human)) | BDBM50503240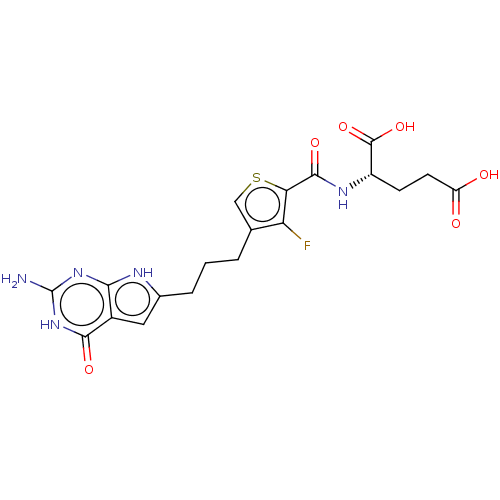 (CHEMBL4445651) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of human PCFT expressed in Chinese hamster R2/PCFT4 cells assessed as reduction in [3H]MTX uptake at pH 5.5 measured over 2 mins by Dixon ... | J Med Chem 61: 4228-4248 (2018) Article DOI: 10.1021/acs.jmedchem.8b00408 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proton-coupled folate transporter (Homo sapiens (Human)) | BDBM50503240 (CHEMBL4445651) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Binding affinity to human PCFT expressed in Chinese hamster R2/PCFT4 cells assessed as antiproliferative activity measured as reduction in cell growt... | J Med Chem 61: 4228-4248 (2018) Article DOI: 10.1021/acs.jmedchem.8b00408 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proton-coupled folate transporter (Homo sapiens (Human)) | BDBM50503240 (CHEMBL4445651) | Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Binding affinity to human PCFT4 expressed in human HeLa R1-11 cells assessed as antiproliferative activity measured as reduction in cell growth after... | J Med Chem 61: 4228-4248 (2018) Article DOI: 10.1021/acs.jmedchem.8b00408 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folate receptor beta (Homo sapiens (Human)) | BDBM50503240 (CHEMBL4445651) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Binding affinity to human FR2 expressed in human HeLa R1-11 cells assessed as antiproliferative activity measured as reduction in cell growth after 9... | J Med Chem 61: 4228-4248 (2018) Article DOI: 10.1021/acs.jmedchem.8b00408 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folate receptor beta (Homo sapiens (Human)) | BDBM50503240 (CHEMBL4445651) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Binding affinity to human FRbeta expressed in Chinese hamster D4 cells assessed as antiproliferative activity measured as reduction in cell growth af... | J Med Chem 61: 4228-4248 (2018) Article DOI: 10.1021/acs.jmedchem.8b00408 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folate receptor alpha (Homo sapiens (Human)) | BDBM50503240 (CHEMBL4445651) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Binding affinity to human FRalpha expressed in Chinese hamster RT16 cells assessed as antiproliferative activity measured as reduction in cell growth... | J Med Chem 61: 4228-4248 (2018) Article DOI: 10.1021/acs.jmedchem.8b00408 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folate transporter 1 (Homo sapiens (Human)) | BDBM50503240 (CHEMBL4445651) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Binding affinity to human RFC2 expressed in human HeLa R1-11 cells assessed as antiproliferative activity measured as reduction in cell growth after ... | J Med Chem 61: 4228-4248 (2018) Article DOI: 10.1021/acs.jmedchem.8b00408 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase/GAR transformylase/AICAR transformylase (Homo sapiens (Human)) | BDBM50503240 (CHEMBL4445651) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Inhibition of GARFTase in human IGROV1 cells assessed as decrease in incorporation of [14C(U)glycine into [14C]formyl GAR formation after 24 hrs | J Med Chem 61: 4228-4248 (2018) Article DOI: 10.1021/acs.jmedchem.8b00408 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folate transporter 1 (Homo sapiens (Human)) | BDBM50503240 (CHEMBL4445651) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University Curated by ChEMBL | Assay Description Binding affinity to human RFC expressed in Chinese hamster PC43-10 cells assessed as antiproliferative activity measured as reduction in cell growth ... | J Med Chem 61: 4228-4248 (2018) Article DOI: 10.1021/acs.jmedchem.8b00408 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||