 Found 13 hits for monomerid = 50504024
Found 13 hits for monomerid = 50504024 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
1,3-beta-glucan synthase component GSC2
(Saccharomyces cerevisiae) | BDBM50504024
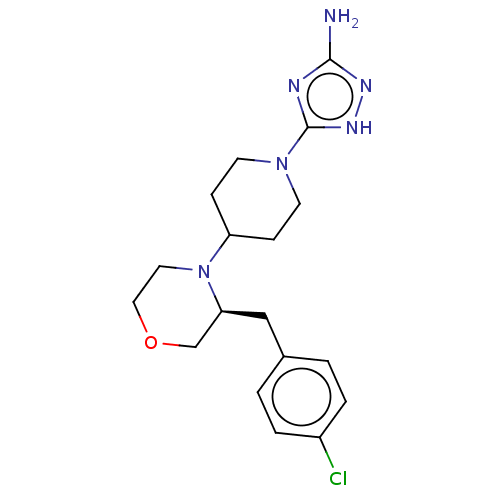
(CHEMBL4461925)Show SMILES Nc1n[nH]c(n1)N1CCC(CC1)N1CCOC[C@@H]1Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H25ClN6O/c19-14-3-1-13(2-4-14)11-16-12-26-10-9-25(16)15-5-7-24(8-6-15)18-21-17(20)22-23-18/h1-4,15-16H,5-12H2,(H3,20,21,22,23)/t16-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA
Curated by ChEMBL
| Assay Description
Displacement of Tracer Red from human ERG by fluorescence polarization assay |
J Med Chem 62: 7126-7145 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00681 |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase component GSC2
(Saccharomyces cerevisiae) | BDBM50504024

(CHEMBL4461925)Show SMILES Nc1n[nH]c(n1)N1CCC(CC1)N1CCOC[C@@H]1Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H25ClN6O/c19-14-3-1-13(2-4-14)11-16-12-26-10-9-25(16)15-5-7-24(8-6-15)18-21-17(20)22-23-18/h1-4,15-16H,5-12H2,(H3,20,21,22,23)/t16-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA
Curated by ChEMBL
| Assay Description
Inhibition of human ERG expressed in CHO-K1 cells by automated Patch Clamp method |
J Med Chem 62: 7126-7145 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00681 |
More data for this
Ligand-Target Pair | |
Chitotriosidase-1
(Homo sapiens (Human)) | BDBM50504024

(CHEMBL4461925)Show SMILES Nc1n[nH]c(n1)N1CCC(CC1)N1CCOC[C@@H]1Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H25ClN6O/c19-14-3-1-13(2-4-14)11-16-12-26-10-9-25(16)15-5-7-24(8-6-15)18-21-17(20)22-23-18/h1-4,15-16H,5-12H2,(H3,20,21,22,23)/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA
Curated by ChEMBL
| Assay Description
Inhibition of full-length C-terminal his-tagged human CHIT1 expressed in CHOK1 cells assessed as reduction in chitinolytic activity using 4-methylumb... |
J Med Chem 62: 7126-7145 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00681 |
More data for this
Ligand-Target Pair | |
Acidic mammalian chitinase
(Homo sapiens (Human)) | BDBM50504024

(CHEMBL4461925)Show SMILES Nc1n[nH]c(n1)N1CCC(CC1)N1CCOC[C@@H]1Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H25ClN6O/c19-14-3-1-13(2-4-14)11-16-12-26-10-9-25(16)15-5-7-24(8-6-15)18-21-17(20)22-23-18/h1-4,15-16H,5-12H2,(H3,20,21,22,23)/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA
Curated by ChEMBL
| Assay Description
Inhibition of full-length C-terminal his-tagged human acidic mammalian chitinase expressed in CHOK1 cells assessed as reduction in chitinolytic activ... |
J Med Chem 62: 7126-7145 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00681 |
More data for this
Ligand-Target Pair | |
Chitotriosidase-1
(Mus musculus) | BDBM50504024

(CHEMBL4461925)Show SMILES Nc1n[nH]c(n1)N1CCC(CC1)N1CCOC[C@@H]1Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H25ClN6O/c19-14-3-1-13(2-4-14)11-16-12-26-10-9-25(16)15-5-7-24(8-6-15)18-21-17(20)22-23-18/h1-4,15-16H,5-12H2,(H3,20,21,22,23)/t16-/m0/s1 | UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA
Curated by ChEMBL
| Assay Description
Inhibition of full-length C-terminal his-tagged mouse CHIT1 expressed in CHO-K1 cells assessed as reduction in chitinolytic activity using 4-methylum... |
J Med Chem 62: 7126-7145 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00681 |
More data for this
Ligand-Target Pair | |
Acidic mammalian chitinase
(Mus musculus) | BDBM50504024

(CHEMBL4461925)Show SMILES Nc1n[nH]c(n1)N1CCC(CC1)N1CCOC[C@@H]1Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H25ClN6O/c19-14-3-1-13(2-4-14)11-16-12-26-10-9-25(16)15-5-7-24(8-6-15)18-21-17(20)22-23-18/h1-4,15-16H,5-12H2,(H3,20,21,22,23)/t16-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA
Curated by ChEMBL
| Assay Description
Inhibition of full-length C-terminal his-tagged mouse acidic mammalian chitinase expressed in CHO-K1 cells assessed as reduction in chitinolytic acti... |
J Med Chem 62: 7126-7145 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00681 |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase component GSC2
(Saccharomyces cerevisiae) | BDBM50504024

(CHEMBL4461925)Show SMILES Nc1n[nH]c(n1)N1CCC(CC1)N1CCOC[C@@H]1Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H25ClN6O/c19-14-3-1-13(2-4-14)11-16-12-26-10-9-25(16)15-5-7-24(8-6-15)18-21-17(20)22-23-18/h1-4,15-16H,5-12H2,(H3,20,21,22,23)/t16-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Rattus norvegicus (rat)) | BDBM50504024

(CHEMBL4461925)Show SMILES Nc1n[nH]c(n1)N1CCC(CC1)N1CCOC[C@@H]1Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H25ClN6O/c19-14-3-1-13(2-4-14)11-16-12-26-10-9-25(16)15-5-7-24(8-6-15)18-21-17(20)22-23-18/h1-4,15-16H,5-12H2,(H3,20,21,22,23)/t16-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA
Curated by ChEMBL
| Assay Description
Displacement of [3H]DA from rat dopamine transporter after 15 mins by scintillation counting analysis |
J Med Chem 62: 7126-7145 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00681 |
More data for this
Ligand-Target Pair | |
Chitotriosidase-1
(Homo sapiens (Human)) | BDBM50504024

(CHEMBL4461925)Show SMILES Nc1n[nH]c(n1)N1CCC(CC1)N1CCOC[C@@H]1Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H25ClN6O/c19-14-3-1-13(2-4-14)11-16-12-26-10-9-25(16)15-5-7-24(8-6-15)18-21-17(20)22-23-18/h1-4,15-16H,5-12H2,(H3,20,21,22,23)/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Chitotriosidase-1
(Mus musculus) | BDBM50504024

(CHEMBL4461925)Show SMILES Nc1n[nH]c(n1)N1CCC(CC1)N1CCOC[C@@H]1Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H25ClN6O/c19-14-3-1-13(2-4-14)11-16-12-26-10-9-25(16)15-5-7-24(8-6-15)18-21-17(20)22-23-18/h1-4,15-16H,5-12H2,(H3,20,21,22,23)/t16-/m0/s1 | UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acidic mammalian chitinase
(Homo sapiens (Human)) | BDBM50504024

(CHEMBL4461925)Show SMILES Nc1n[nH]c(n1)N1CCC(CC1)N1CCOC[C@@H]1Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H25ClN6O/c19-14-3-1-13(2-4-14)11-16-12-26-10-9-25(16)15-5-7-24(8-6-15)18-21-17(20)22-23-18/h1-4,15-16H,5-12H2,(H3,20,21,22,23)/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Acidic mammalian chitinase
(Mus musculus) | BDBM50504024

(CHEMBL4461925)Show SMILES Nc1n[nH]c(n1)N1CCC(CC1)N1CCOC[C@@H]1Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H25ClN6O/c19-14-3-1-13(2-4-14)11-16-12-26-10-9-25(16)15-5-7-24(8-6-15)18-21-17(20)22-23-18/h1-4,15-16H,5-12H2,(H3,20,21,22,23)/t16-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Sodium-dependent dopamine transporter
(Homo sapiens (Human)) | BDBM50504024

(CHEMBL4461925)Show SMILES Nc1n[nH]c(n1)N1CCC(CC1)N1CCOC[C@@H]1Cc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C18H25ClN6O/c19-14-3-1-13(2-4-14)11-16-12-26-10-9-25(16)15-5-7-24(8-6-15)18-21-17(20)22-23-18/h1-4,15-16H,5-12H2,(H3,20,21,22,23)/t16-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
OncoArendi Therapeutics SA
Curated by ChEMBL
| Assay Description
Inhibition of radioligand binding to human dopamine transporter by radioligand binding assay |
J Med Chem 62: 7126-7145 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00681 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data