

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50505789 CHEMBL4471621
SMILES: COc1ccc(cc1)[C@@H]1N(CCc2sccc12)C(=O)Nc1cc(Cl)cc(Cl)c1
InChI Key: InChIKey=VPDBRKAZMIEIGB-FQEVSTJZSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Luteinizing hormone/Choriogonadotropin receptor (Rattus norvegicus) | BDBM50505789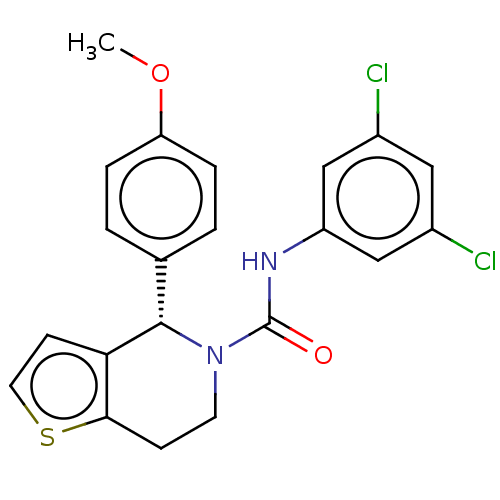 (CHEMBL4471621) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 412 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at rat luteinizing hormone receptor expressed in rat ovary granulosa GLHR15 cell suspension assessed as reduction in agonist-indu... | J Med Chem 62: 10321-10341 (2019) Article DOI: 10.1021/acs.jmedchem.9b01382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C11 (Rattus norvegicus) | BDBM50505789 (CHEMBL4471621) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human thyroid stimulating hormone receptor expressed in rat HTC133 cells assessed as reduction in agonist-induced cAMP product... | J Med Chem 62: 10321-10341 (2019) Article DOI: 10.1021/acs.jmedchem.9b01382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lutropin-choriogonadotropic hormone receptor (Homo sapiens (Human)) | BDBM50505789 (CHEMBL4471621) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 403 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human luteinizing hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins followed ... | J Med Chem 62: 10321-10341 (2019) Article DOI: 10.1021/acs.jmedchem.9b01382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Follicle stimulating hormone receptor (Homo sapiens (Human)) | BDBM50505789 (CHEMBL4471621) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at human follicle stimulating hormone receptor assessed as reduction in agonist-induced cAMP production preincubated for 20 mins ... | J Med Chem 62: 10321-10341 (2019) Article DOI: 10.1021/acs.jmedchem.9b01382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Lutropin-choriogonadotropic hormone receptor (Mus musculus) | BDBM50505789 (CHEMBL4471621) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Antagonist activity at luteinizing hormone receptor in mouse MLTC-1 cells assessed as reduction in agonist-induced cAMP production preincubated for 3... | J Med Chem 62: 10321-10341 (2019) Article DOI: 10.1021/acs.jmedchem.9b01382 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||