

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50509071 CHEMBL4455324
SMILES: OC(=O)C(F)(F)F.OC(=O)C(F)(F)F.CC(C)(C)CNc1nccc(n1)N1CC[C@@H](N)C1
InChI Key: InChIKey=OUXHKDXZJVBYCT-YQFADDPSSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histamine receptor (H3 and H4) (Homo sapiens (Human)) | BDBM50509071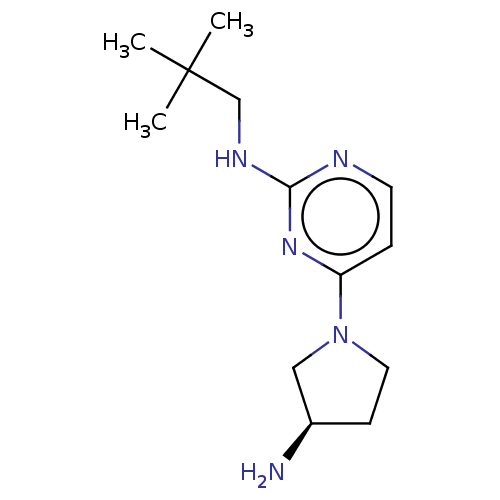 (CHEMBL4455324) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of 10 nM [3H]-histamine from human H4R expressed in Sf9 cell membranes co-expressing Gia2 and beta1gamma2 by competition binding assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H3 receptor (Homo sapiens (Human)) | BDBM50509071 (CHEMBL4455324) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of 3 nM [3H]Nalpha-methylhistamine from human H3R expressed in Sf9 cell membranes co-expressing Gia2 and beta1gamma2 by competition bind... | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50509071 (CHEMBL4455324) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of 5 nM [3H-pyrilamine from human H1R expressed in Sf9 cell membranes co-expressing RGS4 by competition binding assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H2 receptor (Homo sapiens (Human)) | BDBM50509071 (CHEMBL4455324) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of 20 nM [3H]UR-DE257 from human H2R expressed in Sf9 cell membranes co-expressing GsalphaS by competition binding assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 Receptor (Mus musculus (mouse)) | BDBM50509071 (CHEMBL4455324) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 135 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at mouse H4R expressed in HEK293T-beta-arr2-mH4R cells by beta-arrestin2 recruitment assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 Receptor (Rattus norvegicus (rat)) | BDBM50509071 (CHEMBL4455324) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at rat H4R expressed in HEK293-SF-rH4R-His6-CRE-Luc cells incubated for 5 hrs by luciferase reporter gene assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 Receptor (Mus musculus (mouse)) | BDBM50509071 (CHEMBL4455324) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at mouse H4R expressed in HEK293-SF-mH4R-His6-CRE-Luc cells incubated for 5 hrs by luciferase reporter gene assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor (H3 and H4) (Homo sapiens (Human)) | BDBM50509071 (CHEMBL4455324) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4R expressed in HEK293T-beta-arr2-hH4R cells by beta-arrestin2 recruitment assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine H4 Receptor (Rattus norvegicus (rat)) | BDBM50509071 (CHEMBL4455324) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 34 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at rat H4R expressed in HEK293T-beta-arr2-rH4R cells by beta-arrestin2 recruitment assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histamine receptor (H3 and H4) (Homo sapiens (Human)) | BDBM50509071 (CHEMBL4455324) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Agonist activity at human H4R expressed in HEK293-SF-hH4R-His6-CRE-Luc cells incubated for 5 hrs by luciferase reporter gene assay | J Med Chem 62: 8338-8356 (2019) Article DOI: 10.1021/acs.jmedchem.9b01342 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||