

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: COc1ccccc1S(=O)(=O)Nc1ccc(C)cc1Oc1ccc(cc1)C(N)=N
InChI Key: InChIKey=PYYIGZSQKIBTPL-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50509099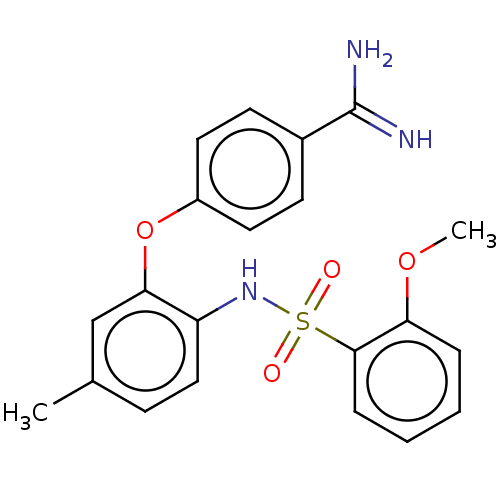 (CHEMBL4565294) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.27E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from mu opioid receptor in rat brain after 60 mins by liquid scintillation counting method | J Med Chem 62: 8631-8641 (2019) Article DOI: 10.1021/acs.jmedchem.9b01003 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50509099 (CHEMBL4565294) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from delta opioid receptor in rat brain after 60 mins by liquid scintillation counting method | J Med Chem 62: 8631-8641 (2019) Article DOI: 10.1021/acs.jmedchem.9b01003 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Rattus norvegicus (rat)) | BDBM50509099 (CHEMBL4565294) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.43E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]U-69,593 from kappa opioid receptor in rat brain after 60 mins by liquid scintillation counting method | J Med Chem 62: 8631-8641 (2019) Article DOI: 10.1021/acs.jmedchem.9b01003 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mas-related G-protein coupled receptor member X1 (Homo sapiens (Human)) | BDBM50509099 (CHEMBL4565294) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
Eisai Co., Ltd. Curated by ChEMBL | Assay Description Agonist activity at human MrgprX1 expressed in HEK293 cells incubated for 2 mins by Fluo4AM dye based FLIPR assay | J Med Chem 62: 8631-8641 (2019) Article DOI: 10.1021/acs.jmedchem.9b01003 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||