

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50510095 CHEMBL4465694
SMILES: [#6]\[#6](-[#6])=[#6]\[#6]-c1c(-[#8])cc(-[#8])c(-[#6]\[#6]=[#6](\[#6])-[#6])c1\[#6]=[#6]\c1ccc(-[#8])cc1
InChI Key: InChIKey=MTVXRRAAXZXGQT-NTEUORMPSA-N
Data: 3 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E synthase/G/H synthase 2 (Homo sapiens (Human)) | BDBM50510095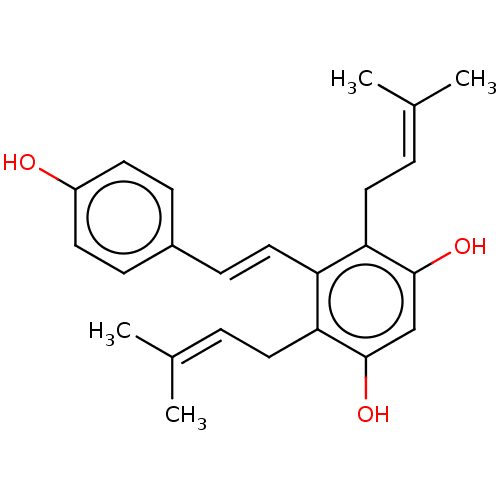 (CHEMBL4465694) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 assessed as reduction in PGE2 production using arachidonic acid substrate by ELISA | J Nat Prod 82: 1839-1848 (2019) Article DOI: 10.1021/acs.jnatprod.9b00081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase (cyclooxygenase) (Ovis aries (Sheep)) | BDBM50510095 (CHEMBL4465694) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of ram seminal vesicle COX1 assessed as reduction in PGE2 production using arachidonic acid substrate by ELISA | J Nat Prod 82: 1839-1848 (2019) Article DOI: 10.1021/acs.jnatprod.9b00081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-lipoxygenase/FLAP (Homo sapiens (Human)) | BDBM50510095 (CHEMBL4465694) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Czech Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LOX assessed as reduction in leukotriene B4 synthesis using arachidonic acid substrate by ELISA | J Nat Prod 82: 1839-1848 (2019) Article DOI: 10.1021/acs.jnatprod.9b00081 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||