

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50510288 CHEMBL4445556
SMILES: NC[C@H](Oc1noc2ccc(cc12)-c1ccccc1Cl)c1ccccc1
InChI Key: InChIKey=HHSLFPVGKCXEOG-FQEVSTJZSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA Gyrase (Escherichia coli (strain K12)) | BDBM50510288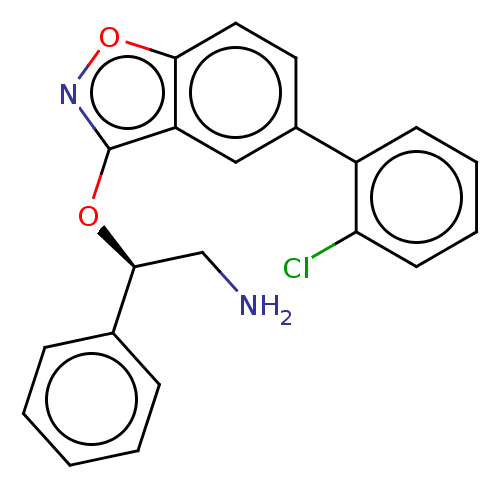 (CHEMBL4445556) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Escherichia coli his-tagged DNA gyrase supercoiling activity using pBR322 DNA in presence of ATP incubated for 1 hr by H19 dye based hi... | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50510288 (CHEMBL4445556) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human ERG by QPatch assay | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Topoisomerase I/II (Homo sapiens (Human)) | BDBM50510288 (CHEMBL4445556) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human DNA topoisomerase 2 alpha using interlinked kDNA as substrate after 30 mins by ethidium bromide staining-based agarose gel electr... | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium channel protein type 5 subunit alpha (Homo sapiens (Human)) | BDBM50510288 (CHEMBL4445556) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of human NaV1.5 expressed in HEK293 cells assessed as decrease in sodium current amplitude at -120 mV holding potential by Qpatch assay | Bioorg Med Chem Lett 29: 1407-1412 (2019) Article DOI: 10.1016/j.bmcl.2019.03.029 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||