

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50510530 CHEMBL4449293
SMILES: C[C@@H]1CCC[C@@](O)(CNC(=O)c2c(Cl)ccc3nc(ccc23)N2CCC(C2)N(C)C)C1
InChI Key: InChIKey=DXYPZGNOLQPKSH-PQXMGZSVSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM50510530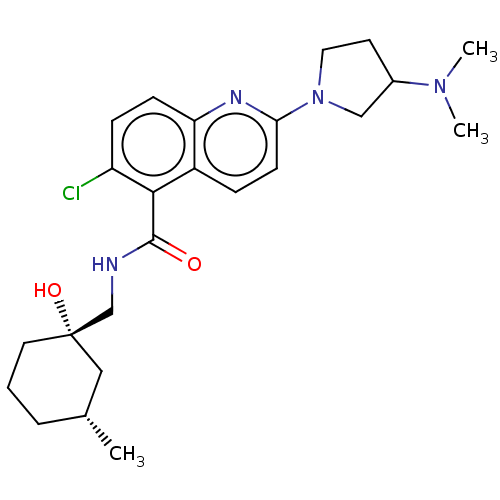 (CHEMBL4449293) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 549 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research and Development Institute Curated by ChEMBL | Assay Description Antagonist activity at rat P2X7 receptor assessed as inhibition of BzATP-induced YO PRO dye uptake preincubated for 20 mins followed by YO PRO dye an... | Bioorg Med Chem Lett 29: 1660-1664 (2019) Article DOI: 10.1016/j.bmcl.2019.04.033 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50510530 (CHEMBL4449293) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research and Development Institute Curated by ChEMBL | Assay Description Antagonist activity at P2X7R in LPS-stimulated human THP1 cells assessed as inhibition of BzATP-induced IL-1beta release preincubated for 30 mins fol... | Bioorg Med Chem Lett 29: 1660-1664 (2019) Article DOI: 10.1016/j.bmcl.2019.04.033 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50510530 (CHEMBL4449293) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research and Development Institute Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced YO PRO dye uptake preincubated for 20 mi... | Bioorg Med Chem Lett 29: 1660-1664 (2019) Article DOI: 10.1016/j.bmcl.2019.04.033 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Mus musculus) | BDBM50510530 (CHEMBL4449293) | KEGG UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research and Development Institute Curated by ChEMBL | Assay Description Antagonist activity at mouse P2X7 receptor assessed as inhibition of BzATP-induced YO PRO dye uptake preincubated for 20 mins followed by YO PRO dye ... | Bioorg Med Chem Lett 29: 1660-1664 (2019) Article DOI: 10.1016/j.bmcl.2019.04.033 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||