

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50511552 CHEMBL4439372
SMILES: CN1C(=O)C2(CCN(CC2)c2nc3N(C)CCCc3c(=O)[nH]2)c2c1cc(F)cc2F
InChI Key: InChIKey=FAANBVRTVUGZKM-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Poly [ADP-ribose] polymerase tankyrase-1 (Homo sapiens (Human)) | BDBM50511552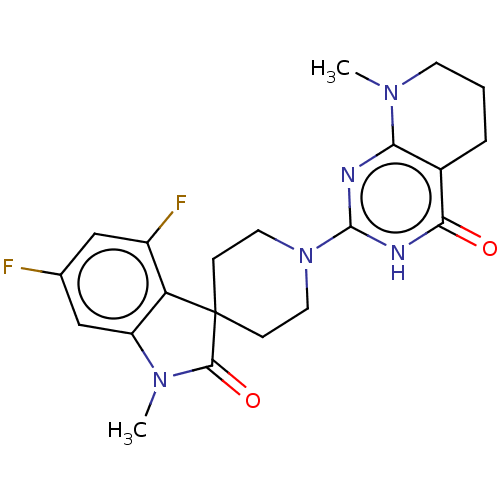 (CHEMBL4439372) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Japanese Foundation for Cancer Research Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal TEV cleavage site-fused/FLAG-poly his-tagged TNKS SAM-PARP domain (1024 to 1327 residues) expressed in Esc... | J Med Chem 63: 4183-4204 (2020) Article DOI: 10.1021/acs.jmedchem.0c00045 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tankyrase 1/2 (Homo sapiens (Human)) | BDBM50511552 (CHEMBL4439372) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Japanese Foundation for Cancer Research Curated by ChEMBL | Assay Description Inhibition of TNKS/TNKS2 (unknown origin) expressed in human DLD1 cells assessed as reduction in Wnt-signaling measured after 24 hrs by TCF-luciferas... | J Med Chem 63: 4183-4204 (2020) Article DOI: 10.1021/acs.jmedchem.0c00045 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tankyrase 1/2 (Homo sapiens (Human)) | BDBM50511552 (CHEMBL4439372) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Japanese Foundation for Cancer Research Curated by ChEMBL | Assay Description Inhibition of TNKS/TNKS2 (unknown origin) expressed in HEK293 cells assessed as reduction in Wnt-signaling measured after 24 hrs by TCF-luciferase re... | J Med Chem 63: 4183-4204 (2020) Article DOI: 10.1021/acs.jmedchem.0c00045 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Poly [ADP-ribose] polymerase tankyrase-2 (Homo sapiens (Human)) | BDBM50511552 (CHEMBL4439372) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Japanese Foundation for Cancer Research Curated by ChEMBL | Assay Description Inhibition of recombinant human N-terminal TEV cleavage site-fused/FLAG-poly his-tagged TNKS2 ARC5-SAM-PARP domain (613 to 1166 residues) expressed i... | J Med Chem 63: 4183-4204 (2020) Article DOI: 10.1021/acs.jmedchem.0c00045 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||