

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50513 4-(2,6-bis(3,4-dimethylphenyl)-1,3,5,7-tetraoxo-1,2,3,5,6,7-hexahydropyrrolo[3,4-f]isoindole-4-carbonyl)benzoic acid::4-[2,6-bis(3,4-dimethylphenyl)-1,3,5,7-tetraketo-pyrrol[3,4-f]isoindole-8-carbonyl]benzoic acid::4-[2,6-bis(3,4-dimethylphenyl)-1,3,5,7-tetrakis(oxidanylidene)pyrrolo[3,4-f]isoindol-8-yl]carbonylbenzoic acid::4-[2,6-bis(3,4-dimethylphenyl)-1,3,5,7-tetraoxopyrrolo[3,4-f]isoindole-8-carbonyl]benzoic acid::4-[[2,6-bis(3,4-dimethylphenyl)-1,3,5,7-tetraoxo-8-pyrrolo[3,4-f]isoindolyl]-oxomethyl]benzoic acid::CHEMBL1324382::MLS000714384::SMR000274364::cid_3108985
SMILES: Cc1ccc(cc1C)-n1c(=O)c2cc3c(c(C(=O)c4ccc(cc4)C(O)=O)c2c1=O)c(=O)n(-c1ccc(C)c(C)c1)c3=O
InChI Key: InChIKey=HCVOMYUMZYAHRX-UHFFFAOYSA-N
Data: 10 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MKP-3 (Rattus norvegicus) | BDBM50513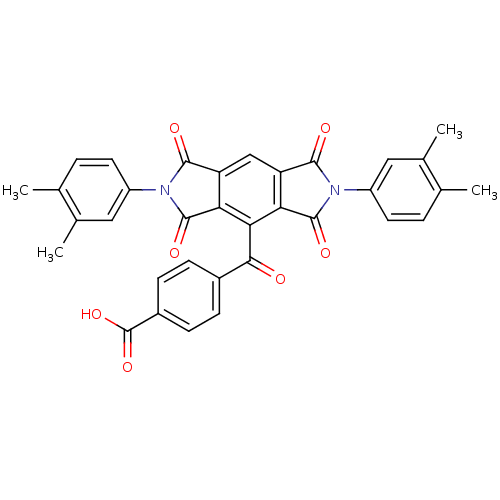 (4-(2,6-bis(3,4-dimethylphenyl)-1,3,5,7-tetraoxo-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.89E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanford-Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2W66J6B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50513 (4-(2,6-bis(3,4-dimethylphenyl)-1,3,5,7-tetraoxo-1,...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University Molecular Libraries Screening Center Curated by PubChem BioAssay | Assay Description NIH Molecular Libraries Screening Centers Network [MLSCN] Emory Chemical Biology Discovery Center in MLSCN Assay provider: Nikolovska-Coleska, Univer... | PubChem Bioassay (2008) BindingDB Entry DOI: 10.7270/Q23X8539 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase (VHR) (Homo sapiens (Human)) | BDBM50513 (4-(2,6-bis(3,4-dimethylphenyl)-1,3,5,7-tetraoxo-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 5.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q29G5K79 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity protein phosphatase (VHR) (Homo sapiens (Human)) | BDBM50513 (4-(2,6-bis(3,4-dimethylphenyl)-1,3,5,7-tetraoxo-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.39E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2009) BindingDB Entry DOI: 10.7270/Q2445JXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50513 (4-(2,6-bis(3,4-dimethylphenyl)-1,3,5,7-tetraoxo-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Low molecular weight phosphotyrosine protein phosphatase (Homo sapiens (Human)) | BDBM50513 (4-(2,6-bis(3,4-dimethylphenyl)-1,3,5,7-tetraoxo-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | >8.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | PubChem Bioassay (2012) BindingDB Entry DOI: 10.7270/Q2GM85XC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Low molecular weight phosphotyrosine protein phosphatase (Homo sapiens (Human)) | BDBM50513 (4-(2,6-bis(3,4-dimethylphenyl)-1,3,5,7-tetraoxo-1,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | PubChem Bioassay (2013) BindingDB Entry DOI: 10.7270/Q2JD4VDZ | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50513 (4-(2,6-bis(3,4-dimethylphenyl)-1,3,5,7-tetraoxo-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO in cell-free system assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase (Homo sapiens (Human)) | BDBM50513 (4-(2,6-bis(3,4-dimethylphenyl)-1,3,5,7-tetraoxo-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of 5-LO in human polymorphonuclear leukocytes assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| carboxy-terminal domain RNA polymerase II polypeptide A small phosphatase 1 isoform 1 (Homo sapiens (Human)) | BDBM50513 (4-(2,6-bis(3,4-dimethylphenyl)-1,3,5,7-tetraoxo-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PCBioAssay | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Burnham Center for Chemical Genomics Curated by PubChem BioAssay | Assay Description Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C... | PubChem Bioassay (2011) BindingDB Entry DOI: 10.7270/Q2V986KM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||