

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50513875 CHEMBL4457468
SMILES: Nc1ccc(cn1)-c1cn(nn1)-c1c(F)ccc(NS(=O)(=O)c2cccc(Cl)c2)c1F
InChI Key: InChIKey=NGSHIRNMRKRWQI-UHFFFAOYSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mixed lineage kinase 7 (Homo sapiens (Human)) | BDBM50513875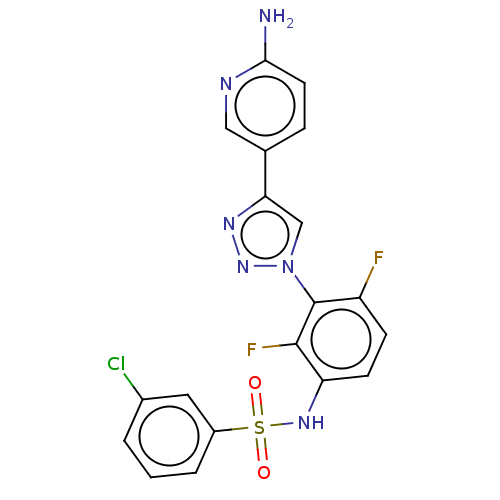 (CHEMBL4457468) | PDB NCI pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Jinan University Curated by ChEMBL | Assay Description Inhibition of N-terminal GST-tagged recombinant human full-length ZAK expressed in baculovirus infected Sf9 insect cells using MBP as substrate after... | J Med Chem 63: 2114-2130 (2020) Article DOI: 10.1021/acs.jmedchem.9b00664 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase B-raf (Homo sapiens (Human)) | BDBM50513875 (CHEMBL4457468) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Jinan University Curated by ChEMBL | Assay Description Inhibition of recombinant human B-RAF V600E mutant using Ser/Thr3 as substrate after 1 hr by FRET-based Z'-Lyte assay | J Med Chem 63: 2114-2130 (2020) Article DOI: 10.1021/acs.jmedchem.9b00664 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||