 Found 10 hits for monomerid = 50513955
Found 10 hits for monomerid = 50513955 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
N-acetyl-beta-D-glucosaminidase (O-GlcNAcase)
(Homo sapiens (Human)) | BDBM50513955
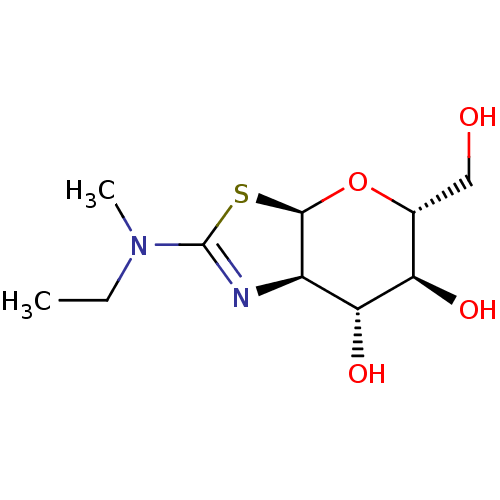
(CHEMBL4483089)Show SMILES [H][C@]12O[C@H](CO)[C@@H](O)[C@H](O)[C@@]1([H])N=C(S2)N(C)CC |r,c:13| Show InChI InChI=1S/C10H18N2O4S/c1-3-12(2)10-11-6-8(15)7(14)5(4-13)16-9(6)17-10/h5-9,13-15H,3-4H2,1-2H3/t5-,6-,7-,8-,9-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human OGA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |
Beta-hexosaminidase subunit beta (Hex B)
(Homo sapiens (Human)) | BDBM50513955

(CHEMBL4483089)Show SMILES [H][C@]12O[C@H](CO)[C@@H](O)[C@H](O)[C@@]1([H])N=C(S2)N(C)CC |r,c:13| Show InChI InChI=1S/C10H18N2O4S/c1-3-12(2)10-11-6-8(15)7(14)5(4-13)16-9(6)17-10/h5-9,13-15H,3-4H2,1-2H3/t5-,6-,7-,8-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of human beta hexosaminidase assessed as inhibitory constant using 4-methylumbelliferyl N-acetyl-beta-D-glucosaminide dihydrate as substra... |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50513955

(CHEMBL4483089)Show SMILES [H][C@]12O[C@H](CO)[C@@H](O)[C@H](O)[C@@]1([H])N=C(S2)N(C)CC |r,c:13| Show InChI InChI=1S/C10H18N2O4S/c1-3-12(2)10-11-6-8(15)7(14)5(4-13)16-9(6)17-10/h5-9,13-15H,3-4H2,1-2H3/t5-,6-,7-,8-,9-/m1/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |
L-type calcium channel alpha-1c/beta-2/alpha2delta-1
(Homo sapiens (Human)) | BDBM50513955

(CHEMBL4483089)Show SMILES [H][C@]12O[C@H](CO)[C@@H](O)[C@H](O)[C@@]1([H])N=C(S2)N(C)CC |r,c:13| Show InChI InChI=1S/C10H18N2O4S/c1-3-12(2)10-11-6-8(15)7(14)5(4-13)16-9(6)17-10/h5-9,13-15H,3-4H2,1-2H3/t5-,6-,7-,8-,9-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of Cav1.2 (unknown origin) |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50513955

(CHEMBL4483089)Show SMILES [H][C@]12O[C@H](CO)[C@@H](O)[C@H](O)[C@@]1([H])N=C(S2)N(C)CC |r,c:13| Show InChI InChI=1S/C10H18N2O4S/c1-3-12(2)10-11-6-8(15)7(14)5(4-13)16-9(6)17-10/h5-9,13-15H,3-4H2,1-2H3/t5-,6-,7-,8-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |
Pregnane X receptor
(Homo sapiens (Human)) | BDBM50513955

(CHEMBL4483089)Show SMILES [H][C@]12O[C@H](CO)[C@@H](O)[C@H](O)[C@@]1([H])N=C(S2)N(C)CC |r,c:13| Show InChI InChI=1S/C10H18N2O4S/c1-3-12(2)10-11-6-8(15)7(14)5(4-13)16-9(6)17-10/h5-9,13-15H,3-4H2,1-2H3/t5-,6-,7-,8-,9-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Activation of PXR (unknown origin) assessed as CYP3A4 induction |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50513955

(CHEMBL4483089)Show SMILES [H][C@]12O[C@H](CO)[C@@H](O)[C@H](O)[C@@]1([H])N=C(S2)N(C)CC |r,c:13| Show InChI InChI=1S/C10H18N2O4S/c1-3-12(2)10-11-6-8(15)7(14)5(4-13)16-9(6)17-10/h5-9,13-15H,3-4H2,1-2H3/t5-,6-,7-,8-,9-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase component GLS2
(Saccharomyces cerevisiae) | BDBM50513955

(CHEMBL4483089)Show SMILES [H][C@]12O[C@H](CO)[C@@H](O)[C@H](O)[C@@]1([H])N=C(S2)N(C)CC |r,c:13| Show InChI InChI=1S/C10H18N2O4S/c1-3-12(2)10-11-6-8(15)7(14)5(4-13)16-9(6)17-10/h5-9,13-15H,3-4H2,1-2H3/t5-,6-,7-,8-,9-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Displacement of MK-0499 from human ERG |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |
Protein O-GlcNAcase
(Rattus norvegicus) | BDBM50513955

(CHEMBL4483089)Show SMILES [H][C@]12O[C@H](CO)[C@@H](O)[C@H](O)[C@@]1([H])N=C(S2)N(C)CC |r,c:13| Show InChI InChI=1S/C10H18N2O4S/c1-3-12(2)10-11-6-8(15)7(14)5(4-13)16-9(6)17-10/h5-9,13-15H,3-4H2,1-2H3/t5-,6-,7-,8-,9-/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 37 | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of OGA in rat PC12 cells assessed as OGlcNAcylated protein level incubated for 24 hrs by ELISA |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |
Sodium channel protein type 5 subunit alpha
(Homo sapiens (Human)) | BDBM50513955

(CHEMBL4483089)Show SMILES [H][C@]12O[C@H](CO)[C@@H](O)[C@H](O)[C@@]1([H])N=C(S2)N(C)CC |r,c:13| Show InChI InChI=1S/C10H18N2O4S/c1-3-12(2)10-11-6-8(15)7(14)5(4-13)16-9(6)17-10/h5-9,13-15H,3-4H2,1-2H3/t5-,6-,7-,8-,9-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Inhibition of Nav1.5 (unknown origin) |
J Med Chem 62: 10062-10097 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01090 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data