

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50514858 CHEMBL4577486
SMILES: CN1CCc2c(C1)nnn2CC(=O)Nc1ccc(Cl)c(c1)S(=O)(=O)N1CC[C@@H](C1)C1CC1
InChI Key: InChIKey=DBLNLPCEKQBLAI-HNNXBMFYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone acetyltransferase KAT2A/KAT2B (Homo sapiens (Human)) | BDBM50514858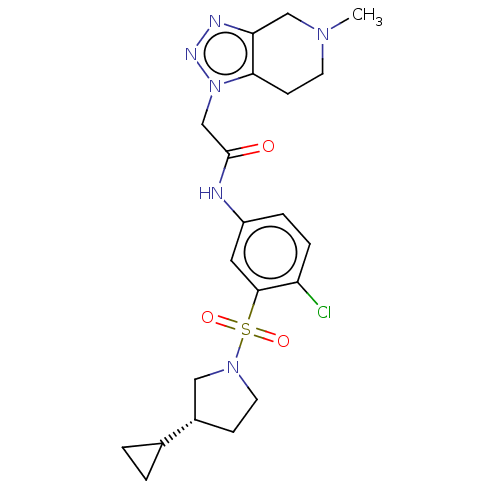 (CHEMBL4577486) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 501 | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to human partial length GCN5L2 (E726 to K837 residues) expressed in bacterial expression system by BROMOscan assay | J Med Chem 63: 5212-5241 (2020) Article DOI: 10.1021/acs.jmedchem.0c00021 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cat eye syndrome critical region protein 2 (Homo sapiens (Human)) | BDBM50514858 (CHEMBL4577486) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Inhibition of BSP-BODIPY binding to Nanoluc-tagged CECR2 (unknown origin) expressed in HEK293 cells measured after 4 to 6 hrs by NanoBRET assay | J Med Chem 63: 5212-5241 (2020) Article DOI: 10.1021/acs.jmedchem.0c00021 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM50514858 (CHEMBL4577486) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Inhibition of TAF1 bromodomain 2 (unknown origin) | J Med Chem 63: 5212-5241 (2020) Article DOI: 10.1021/acs.jmedchem.0c00021 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ATPase family AAA domain-containing protein 2 (Homo sapiens (Human)) | BDBM50514858 (CHEMBL4577486) | PDB MMDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Displacement of Alexa Fluor 488-labeled ligand from FLAG-6His-Tev-fused ATAD2 (981 to 1121 residue) (unknown origin) measured after 30 mins by TR-FRE... | J Med Chem 63: 5212-5241 (2020) Article DOI: 10.1021/acs.jmedchem.0c00021 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Transcription initiation factor TFIID subunit 1 (Homo sapiens (Human)) | BDBM50514858 (CHEMBL4577486) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to human partial length TAF1 bromodomain 2 (D1521 to D1656 residues) expressed in bacterial expression system by BROMOscan assay | J Med Chem 63: 5212-5241 (2020) Article DOI: 10.1021/acs.jmedchem.0c00021 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50514858 (CHEMBL4577486) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Displacement of Alexa Fluor 647-labeled ligand from BRD4 BD2 (unknown origin) measured after 30 mins by TR-FRET assay | J Med Chem 63: 5212-5241 (2020) Article DOI: 10.1021/acs.jmedchem.0c00021 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50514858 (CHEMBL4577486) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Displacement of Alexa Fluor 647-labeled ligand from BRD4 BD1 Y390A mutant (1 to 477 residue) (unknown origin) measured after 30 mins by TR-FRET assay | J Med Chem 63: 5212-5241 (2020) Article DOI: 10.1021/acs.jmedchem.0c00021 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cat eye syndrome critical region protein 2 (Homo sapiens (Human)) | BDBM50514858 (CHEMBL4577486) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Displacement of Alexa Fluor 647-labeled GSK tracer from FLAG-6His-Tev-fused CECR2 (424 to 543 residue) (unknown origin) measured after 15 mins by TR-... | J Med Chem 63: 5212-5241 (2020) Article DOI: 10.1021/acs.jmedchem.0c00021 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cat eye syndrome critical region protein 2 (Homo sapiens (Human)) | BDBM50514858 (CHEMBL4577486) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a |
University of Strathclyde Curated by ChEMBL | Assay Description Binding affinity to human partial length CECR2 (P423 to D543 residues) expressed in bacterial expression system by BROMOscan assay | J Med Chem 63: 5212-5241 (2020) Article DOI: 10.1021/acs.jmedchem.0c00021 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||