

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: CC1(C)Cc2cc(NC(=O)c3cnn4cccnc34)c(cc2O1)N1CCN(CC(N)=O)CC1
InChI Key: InChIKey=TVWUXEGJFJCUJM-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50514949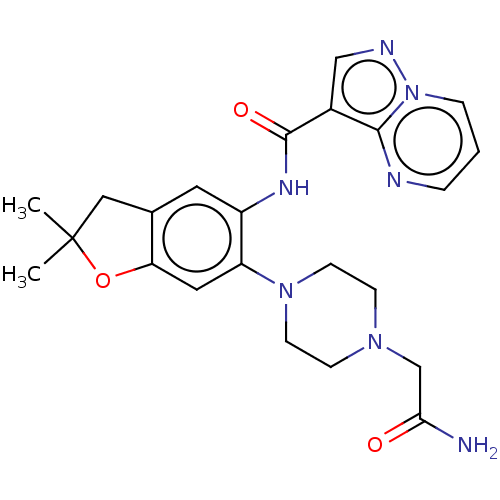 (CHEMBL4483551 | US10988478, Example 187) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc. US Patent | Assay Description Kinase activities were assayed using the Transcreener-Fluorecescence polarization platform (BelBrook Labs, Madison, Wis., USA) that measures amounts ... | US Patent US10988478 (2021) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50514949 (CHEMBL4483551 | US10988478, Example 187) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of human recombinant full length His6-tagged IRAK4 expressed in baculovirus expression system using H-KKARFSRFAGSSPSQSSMVAR as substrate i... | J Med Chem 62: 6223-6240 (2019) Article DOI: 10.1021/acs.jmedchem.9b00439 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interleukin-1 receptor-associated kinase 4 (Homo sapiens (Human)) | BDBM50514949 (CHEMBL4483551 | US10988478, Example 187) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech Curated by ChEMBL | Assay Description Inhibition of IRAK4 in human THP1-Xblue-MD2-CD14 cells assessed as reduction in LPS-induced NFkappaB transcription by measuring alkaline phosphatase ... | J Med Chem 62: 6223-6240 (2019) Article DOI: 10.1021/acs.jmedchem.9b00439 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||