

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50517185 CHEMBL4466759
SMILES: Fc1cccc2CN(CCc12)C(=O)c1cn(CC(=O)NC2CN(C2)C(=O)C=C)c2ccc(Br)cc12
InChI Key: InChIKey=YYNAVMPHBCXAST-UHFFFAOYSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GTPase KRas (Homo sapiens (Human)) | BDBM50517185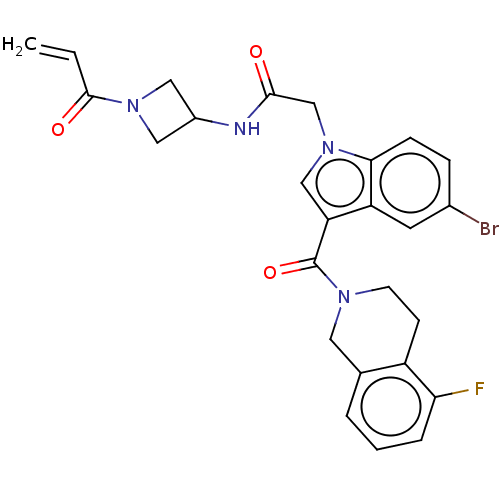 (CHEMBL4466759 | US11053226, Example 118) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of KRAS G12C mutant in human MIAPaCa2 cells assessed as reduction in EGF-induced ERK1/2 phosphorylation incubated for 4 hrs followed by EG... | ACS Med Chem Lett 10: 1302-1308 (2019) Article DOI: 10.1021/acsmedchemlett.9b00258 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50517185 (CHEMBL4466759 | US11053226, Example 118) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50517185 (CHEMBL4466759 | US11053226, Example 118) | PDB GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GTPase KRas (Homo sapiens (Human)) | BDBM50517185 (CHEMBL4466759 | US11053226, Example 118) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of GDP bound N terminal His-tagged KRAS G12C/C118A mutant (unknown origin) (1 to 169 residues) assessed as reduction in SOS-mediated guani... | ACS Med Chem Lett 10: 1302-1308 (2019) Article DOI: 10.1021/acsmedchemlett.9b00258 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||