 Found 15 hits for monomerid = 50520151
Found 15 hits for monomerid = 50520151 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein cereblon/Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50520151
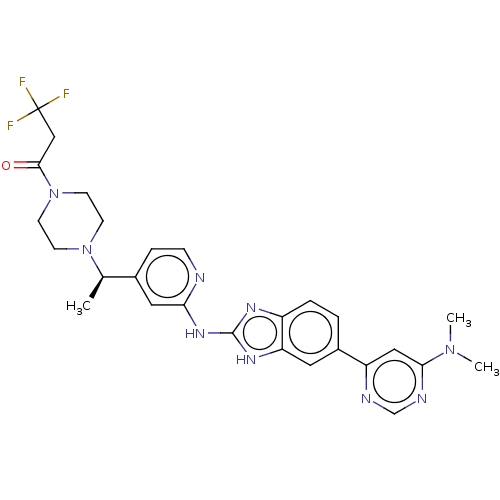
(CHEMBL4537673)Show SMILES C[C@@H](N1CCN(CC1)C(=O)CC(F)(F)F)c1ccnc(Nc2nc3ccc(cc3[nH]2)-c2cc(ncn2)N(C)C)c1 |r| Show InChI InChI=1S/C27H30F3N9O/c1-17(38-8-10-39(11-9-38)25(40)15-27(28,29)30)18-6-7-31-23(13-18)36-26-34-20-5-4-19(12-22(20)35-26)21-14-24(37(2)3)33-16-32-21/h4-7,12-14,16-17H,8-11,15H2,1-3H3,(H2,31,34,35,36)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ... |
J Med Chem 63: 601-612 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01460 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50520151

(CHEMBL4537673)Show SMILES C[C@@H](N1CCN(CC1)C(=O)CC(F)(F)F)c1ccnc(Nc2nc3ccc(cc3[nH]2)-c2cc(ncn2)N(C)C)c1 |r| Show InChI InChI=1S/C27H30F3N9O/c1-17(38-8-10-39(11-9-38)25(40)15-27(28,29)30)18-6-7-31-23(13-18)36-26-34-20-5-4-19(12-22(20)35-26)21-14-24(37(2)3)33-16-32-21/h4-7,12-14,16-17H,8-11,15H2,1-3H3,(H2,31,34,35,36)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) measured under preincubated conditions |
J Med Chem 63: 601-612 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01460 |
More data for this
Ligand-Target Pair | |
IKK epsilon/TBK1
(Homo sapiens (Human)) | BDBM50520151

(CHEMBL4537673)Show SMILES C[C@@H](N1CCN(CC1)C(=O)CC(F)(F)F)c1ccnc(Nc2nc3ccc(cc3[nH]2)-c2cc(ncn2)N(C)C)c1 |r| Show InChI InChI=1S/C27H30F3N9O/c1-17(38-8-10-39(11-9-38)25(40)15-27(28,29)30)18-6-7-31-23(13-18)36-26-34-20-5-4-19(12-22(20)35-26)21-14-24(37(2)3)33-16-32-21/h4-7,12-14,16-17H,8-11,15H2,1-3H3,(H2,31,34,35,36)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of IKKepsilon/TBK1 in human MDB-MB-231 cells transduced with lentiviral vector expressing human IRF3 assessed as decrease in poly(I:C)-sti... |
J Med Chem 63: 601-612 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01460 |
More data for this
Ligand-Target Pair | |
Inhibitor of nuclear factor kappa-B kinase subunit epsilon
(Homo sapiens (Human)) | BDBM50520151

(CHEMBL4537673)Show SMILES C[C@@H](N1CCN(CC1)C(=O)CC(F)(F)F)c1ccnc(Nc2nc3ccc(cc3[nH]2)-c2cc(ncn2)N(C)C)c1 |r| Show InChI InChI=1S/C27H30F3N9O/c1-17(38-8-10-39(11-9-38)25(40)15-27(28,29)30)18-6-7-31-23(13-18)36-26-34-20-5-4-19(12-22(20)35-26)21-14-24(37(2)3)33-16-32-21/h4-7,12-14,16-17H,8-11,15H2,1-3H3,(H2,31,34,35,36)/t17-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human C-terminal GST-tagged IKKepsilon expressed in baculovirus expression system using biotin-labelled Ahx-GDE... |
J Med Chem 63: 601-612 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01460 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase alpha 6
(Homo sapiens (Human)) | BDBM50520151

(CHEMBL4537673)Show SMILES C[C@@H](N1CCN(CC1)C(=O)CC(F)(F)F)c1ccnc(Nc2nc3ccc(cc3[nH]2)-c2cc(ncn2)N(C)C)c1 |r| Show InChI InChI=1S/C27H30F3N9O/c1-17(38-8-10-39(11-9-38)25(40)15-27(28,29)30)18-6-7-31-23(13-18)36-26-34-20-5-4-19(12-22(20)35-26)21-14-24(37(2)3)33-16-32-21/h4-7,12-14,16-17H,8-11,15H2,1-3H3,(H2,31,34,35,36)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 276 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human His6-tagged RSK4 expressed in baculovirus infected sf21 cells using biotin labelled Ahx-KKLNRTLSFAEPG pep... |
J Med Chem 63: 601-612 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01460 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A
(Homo sapiens (Human)) | BDBM50520151

(CHEMBL4537673)Show SMILES C[C@@H](N1CCN(CC1)C(=O)CC(F)(F)F)c1ccnc(Nc2nc3ccc(cc3[nH]2)-c2cc(ncn2)N(C)C)c1 |r| Show InChI InChI=1S/C27H30F3N9O/c1-17(38-8-10-39(11-9-38)25(40)15-27(28,29)30)18-6-7-31-23(13-18)36-26-34-20-5-4-19(12-22(20)35-26)21-14-24(37(2)3)33-16-32-21/h4-7,12-14,16-17H,8-11,15H2,1-3H3,(H2,31,34,35,36)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
J Med Chem 63: 601-612 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01460 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50520151

(CHEMBL4537673)Show SMILES C[C@@H](N1CCN(CC1)C(=O)CC(F)(F)F)c1ccnc(Nc2nc3ccc(cc3[nH]2)-c2cc(ncn2)N(C)C)c1 |r| Show InChI InChI=1S/C27H30F3N9O/c1-17(38-8-10-39(11-9-38)25(40)15-27(28,29)30)18-6-7-31-23(13-18)36-26-34-20-5-4-19(12-22(20)35-26)21-14-24(37(2)3)33-16-32-21/h4-7,12-14,16-17H,8-11,15H2,1-3H3,(H2,31,34,35,36)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
J Med Chem 63: 601-612 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01460 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50520151

(CHEMBL4537673)Show SMILES C[C@@H](N1CCN(CC1)C(=O)CC(F)(F)F)c1ccnc(Nc2nc3ccc(cc3[nH]2)-c2cc(ncn2)N(C)C)c1 |r| Show InChI InChI=1S/C27H30F3N9O/c1-17(38-8-10-39(11-9-38)25(40)15-27(28,29)30)18-6-7-31-23(13-18)36-26-34-20-5-4-19(12-22(20)35-26)21-14-24(37(2)3)33-16-32-21/h4-7,12-14,16-17H,8-11,15H2,1-3H3,(H2,31,34,35,36)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
J Med Chem 63: 601-612 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01460 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50520151

(CHEMBL4537673)Show SMILES C[C@@H](N1CCN(CC1)C(=O)CC(F)(F)F)c1ccnc(Nc2nc3ccc(cc3[nH]2)-c2cc(ncn2)N(C)C)c1 |r| Show InChI InChI=1S/C27H30F3N9O/c1-17(38-8-10-39(11-9-38)25(40)15-27(28,29)30)18-6-7-31-23(13-18)36-26-34-20-5-4-19(12-22(20)35-26)21-14-24(37(2)3)33-16-32-21/h4-7,12-14,16-17H,8-11,15H2,1-3H3,(H2,31,34,35,36)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
J Med Chem 63: 601-612 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01460 |
More data for this
Ligand-Target Pair | |
Protein cereblon/Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM50520151

(CHEMBL4537673)Show SMILES C[C@@H](N1CCN(CC1)C(=O)CC(F)(F)F)c1ccnc(Nc2nc3ccc(cc3[nH]2)-c2cc(ncn2)N(C)C)c1 |r| Show InChI InChI=1S/C27H30F3N9O/c1-17(38-8-10-39(11-9-38)25(40)15-27(28,29)30)18-6-7-31-23(13-18)36-26-34-20-5-4-19(12-22(20)35-26)21-14-24(37(2)3)33-16-32-21/h4-7,12-14,16-17H,8-11,15H2,1-3H3,(H2,31,34,35,36)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant full length human N-terminal His-tagged TBK1 using biotin-labelled Ahx-GDEDFSSFAEPG peptide as substrate preincubated with ... |
J Med Chem 63: 601-612 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01460 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50520151

(CHEMBL4537673)Show SMILES C[C@@H](N1CCN(CC1)C(=O)CC(F)(F)F)c1ccnc(Nc2nc3ccc(cc3[nH]2)-c2cc(ncn2)N(C)C)c1 |r| Show InChI InChI=1S/C27H30F3N9O/c1-17(38-8-10-39(11-9-38)25(40)15-27(28,29)30)18-6-7-31-23(13-18)36-26-34-20-5-4-19(12-22(20)35-26)21-14-24(37(2)3)33-16-32-21/h4-7,12-14,16-17H,8-11,15H2,1-3H3,(H2,31,34,35,36)/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
J Med Chem 63: 601-612 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01460 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 17A
(Homo sapiens (Human)) | BDBM50520151

(CHEMBL4537673)Show SMILES C[C@@H](N1CCN(CC1)C(=O)CC(F)(F)F)c1ccnc(Nc2nc3ccc(cc3[nH]2)-c2cc(ncn2)N(C)C)c1 |r| Show InChI InChI=1S/C27H30F3N9O/c1-17(38-8-10-39(11-9-38)25(40)15-27(28,29)30)18-6-7-31-23(13-18)36-26-34-20-5-4-19(12-22(20)35-26)21-14-24(37(2)3)33-16-32-21/h4-7,12-14,16-17H,8-11,15H2,1-3H3,(H2,31,34,35,36)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 311 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length DRAK1 (R32 to E363 residues) expressed in bacterial expression system by Kinomescan method |
J Med Chem 63: 601-612 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01460 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase ULK1
(Homo sapiens (Human)) | BDBM50520151

(CHEMBL4537673)Show SMILES C[C@@H](N1CCN(CC1)C(=O)CC(F)(F)F)c1ccnc(Nc2nc3ccc(cc3[nH]2)-c2cc(ncn2)N(C)C)c1 |r| Show InChI InChI=1S/C27H30F3N9O/c1-17(38-8-10-39(11-9-38)25(40)15-27(28,29)30)18-6-7-31-23(13-18)36-26-34-20-5-4-19(12-22(20)35-26)21-14-24(37(2)3)33-16-32-21/h4-7,12-14,16-17H,8-11,15H2,1-3H3,(H2,31,34,35,36)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.93E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of wild-type human partial length ULK1 (M1 to T309 residues) expressed in mammalian expression system by Kinomescan method |
J Med Chem 63: 601-612 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01460 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50520151

(CHEMBL4537673)Show SMILES C[C@@H](N1CCN(CC1)C(=O)CC(F)(F)F)c1ccnc(Nc2nc3ccc(cc3[nH]2)-c2cc(ncn2)N(C)C)c1 |r| Show InChI InChI=1S/C27H30F3N9O/c1-17(38-8-10-39(11-9-38)25(40)15-27(28,29)30)18-6-7-31-23(13-18)36-26-34-20-5-4-19(12-22(20)35-26)21-14-24(37(2)3)33-16-32-21/h4-7,12-14,16-17H,8-11,15H2,1-3H3,(H2,31,34,35,36)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 123 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal GST-tagged FLT3 (564 to end residues) expressed in sf21 cells using biotin labelled Ahx-GGEEEEYFELVKKKK pe... |
J Med Chem 63: 601-612 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01460 |
More data for this
Ligand-Target Pair | |
1,3-beta-glucan synthase component GLS2
(Saccharomyces cerevisiae) | BDBM50520151

(CHEMBL4537673)Show SMILES C[C@@H](N1CCN(CC1)C(=O)CC(F)(F)F)c1ccnc(Nc2nc3ccc(cc3[nH]2)-c2cc(ncn2)N(C)C)c1 |r| Show InChI InChI=1S/C27H30F3N9O/c1-17(38-8-10-39(11-9-38)25(40)15-27(28,29)30)18-6-7-31-23(13-18)36-26-34-20-5-4-19(12-22(20)35-26)21-14-24(37(2)3)33-16-32-21/h4-7,12-14,16-17H,8-11,15H2,1-3H3,(H2,31,34,35,36)/t17-/m1/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
J Med Chem 63: 601-612 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01460 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data