

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50520502 CHEMBL4447903
SMILES: CNC(=O)O[C@@H](CC(C)C)c1nc(cs1)[C@H]1OC(=O)C\C=C\C(\C)=C\[C@@H](O)[C@@H](C)\C=C(\C)/C=C(/C)\C=C\[C@@H](O)[C@H](C)[C@H](O)\C(C)=C\C=C\[C@@H]1C
InChI Key: InChIKey=TVOXGUCNTJQSOX-YXKAYWIHSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A3 (Homo sapiens (Human)) | BDBM50520502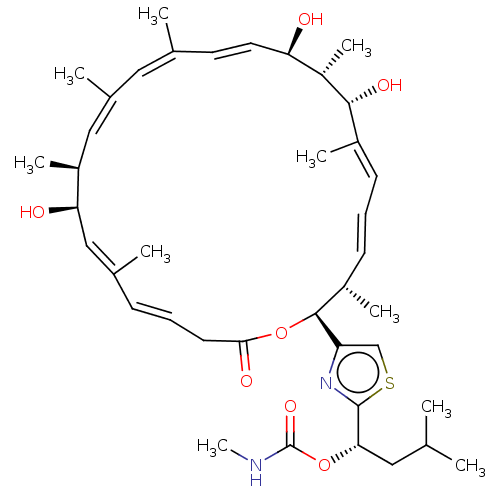 (CHEMBL4447903) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Bonn Curated by ChEMBL | Assay Description Displacement of [3H]PSB-11 from human A3 adenosine receptor expressed in CHO cell membranes incubated for 60 mins by liquid scintillation counting me... | J Med Chem 63: 1684-1698 (2020) Article DOI: 10.1021/acs.jmedchem.9b01887 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 3 (Homo sapiens (Human)) | BDBM50520502 (CHEMBL4447903) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Bonn Curated by ChEMBL | Assay Description Inhibition of human P2X3 assessed as reduction in agonist-induced intracellular Ca2+ concentration pre-incubated for 30 mins before agonist addition ... | J Med Chem 63: 1684-1698 (2020) Article DOI: 10.1021/acs.jmedchem.9b01887 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukocyte elastase (Homo sapiens (Human)) | BDBM50520502 (CHEMBL4447903) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Bonn Curated by ChEMBL | Assay Description Inhibition of human leukocyte elastase assessed as reduction in pNA release using chromogenic MeO-Suc-Ala-Ala-Pro-Val-pNA substrate measured over 10 ... | J Med Chem 63: 1684-1698 (2020) Article DOI: 10.1021/acs.jmedchem.9b01887 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||