

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50523304 CHEMBL4542774
SMILES: Brc1ccc(cc1)-n1cc(COc2ccc(\C=C3/SC(=S)NC3=O)cc2)nn1
InChI Key: InChIKey=AQWUYXBCEWJPSQ-MFOYZWKCSA-N
Data: 2 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E synthase/G/H synthase 2 (Homo sapiens (Human)) | BDBM50523304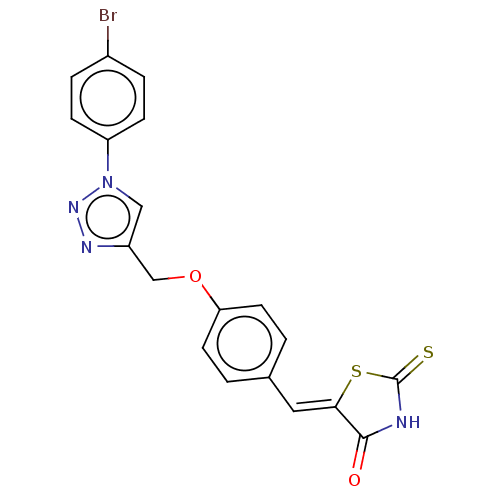 (CHEMBL4542774) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of human recombinant COX2 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition ... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase (cyclooxygenase) (Ovis aries (Sheep)) | BDBM50523304 (CHEMBL4542774) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Alexandria University Curated by ChEMBL | Assay Description Inhibition of ovine COX1 catalytic activity using arachidonic acid as substrate pre-incubated for 10 mins followed by substrate addition and measured... | Eur J Med Chem 167: 562-582 (2019) Article DOI: 10.1016/j.ejmech.2019.02.034 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||