

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50524504 CHEMBL4440982
SMILES: FC(F)(F)C1(NC(Nc2ccccc2Br)=Nc2ccc(Cl)cc12)C#CC1CC1
InChI Key: InChIKey=REDZQXWKFVNPGL-UHFFFAOYSA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50524504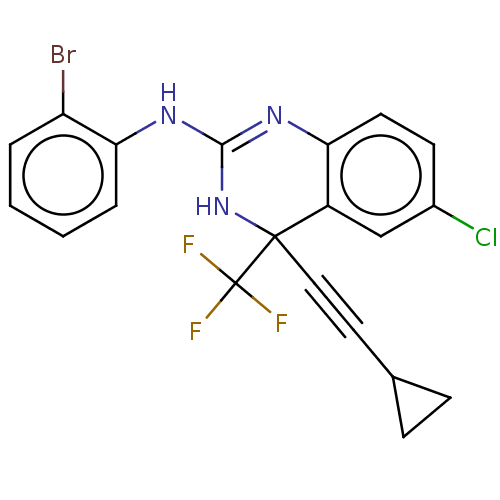 (CHEMBL4440982) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Fudan University Curated by ChEMBL | Assay Description Inhibition of recombinant wild-type HIV1 3B reverse transcriptase assessed as reduction in biotin-dUTP incorporation using poly(rA)/oligo(dT)16 as te... | Eur J Med Chem 176: 11-20 (2019) Article DOI: 10.1016/j.ejmech.2019.05.011 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||