

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50525084 CHEMBL4528032
SMILES: CC(C)[C@@H]1NC(=O)[C@H](Cc2cccs2)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)CCCSC[C@H](NC(=O)[C@H](CC(N)=O)NC1=O)C(=O)N1CCC[C@H]1C(=O)N[C@H](CO)CCCNC(N)=N
InChI Key: InChIKey=VEFXJYXHOJJJST-XVHTWCIUSA-N
Data: 3 EC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxytocin receptor (Homo sapiens (Human)) | BDBM50525084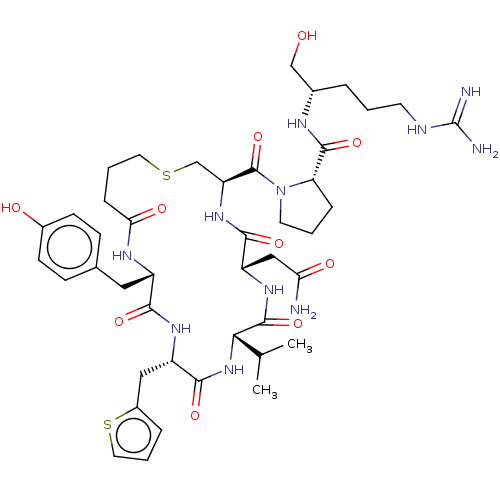 (CHEMBL4528032) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a |
Ferring Research Institute Inc. Curated by ChEMBL | Assay Description Agonist activity at human OTR stably expressed in CHOK1 cells by NFAT-luciferase reporter gene assay | J Med Chem 62: 4991-5005 (2019) Article DOI: 10.1021/acs.jmedchem.9b00132 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50525084 (CHEMBL4528032) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
Ferring Research Institute Inc. Curated by ChEMBL | Assay Description Agonist activity at recombinant human V2 receptor expressed in HEK293 cells measured after 5 hrs by cAMP response element driven luciferase reporter ... | J Med Chem 62: 4991-5005 (2019) Article DOI: 10.1021/acs.jmedchem.9b00132 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (Homo sapiens (Human)) | BDBM50525084 (CHEMBL4528032) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 520 | n/a | n/a | n/a | n/a |
Ferring Research Institute Inc. Curated by ChEMBL | Assay Description Agonist activity at recombinant human V1B receptor expressed in HEK293 cells measured after 5 hrs by luciferase reporter gene assay | J Med Chem 62: 4991-5005 (2019) Article DOI: 10.1021/acs.jmedchem.9b00132 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||