

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50527569 CHEMBL4440098
SMILES: CCS(=O)(=O)Cc1cnc(Oc2ccc(F)cc2F)c(c1)-c1cc(C)c2n1cc[nH]c2=O
InChI Key: InChIKey=UETQFSCWDMJWMM-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50527569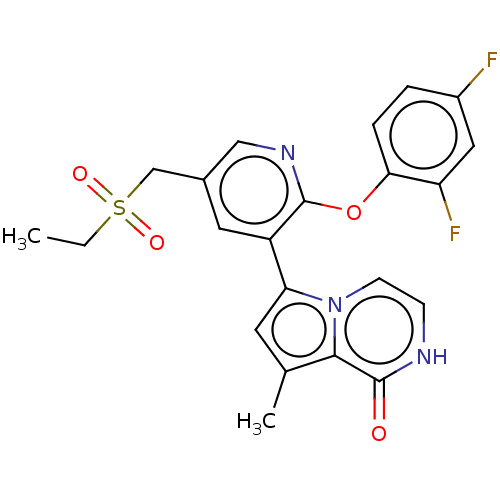 (CHEMBL4440098) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of SGRG-K(Ac)-GG-K(Ac)-GLG-K(Ac)-GGA-K(Ac)-RHRKVGG-K-Biotin binding to N-terminal His6-tagged BRD4 bromodomain 1 (49 to 170 residues) (unk... | J Med Chem 63: 3956-3975 (2020) Article DOI: 10.1021/acs.jmedchem.9b01784 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50527569 (CHEMBL4440098) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Binding affinity to human partial length BRD4 bromodomain 2 long isoform (K333 to E460 residues) expressed in bacterial expression system by BROMOsca... | J Med Chem 63: 3956-3975 (2020) Article DOI: 10.1021/acs.jmedchem.9b01784 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| HIF1A/p300/CREB-binding protein (Homo sapiens (Human)) | BDBM50527569 (CHEMBL4440098) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of MSGRGK(Ac)-GGK(Ac)GLGK(Ac)GGAKRHR-biotin binding to EP300 (1040 to 1160 residues) (unknown origin) expressed in Escherichia coli BL21 (... | J Med Chem 63: 3956-3975 (2020) Article DOI: 10.1021/acs.jmedchem.9b01784 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain and extra-terminal motif (BET) (Homo sapiens (Human)) | BDBM50527569 (CHEMBL4440098) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Binding affinity to human partial length BRD2 BD2 isoform 1 (E348 to D455 residues) expressed in bacterial expression system by bromoscan assay | J Med Chem 63: 3956-3975 (2020) Article DOI: 10.1021/acs.jmedchem.9b01784 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain and extra-terminal motif (BET) (Homo sapiens (Human)) | BDBM50527569 (CHEMBL4440098) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Binding affinity to human partial length BRD3 BD2 (G306 to P416 residues) expressed in bacterial expression system by bromoscan assay | J Med Chem 63: 3956-3975 (2020) Article DOI: 10.1021/acs.jmedchem.9b01784 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A (Homo sapiens (Human)) | BDBM50527569 (CHEMBL4440098) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate preincubated for 10 mins followed by NADPH addition and measured after 1... | J Med Chem 63: 3956-3975 (2020) Article DOI: 10.1021/acs.jmedchem.9b01784 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50527569 (CHEMBL4440098) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate preincubated for 10 mins followed by NADPH addition and measured after 15... | J Med Chem 63: 3956-3975 (2020) Article DOI: 10.1021/acs.jmedchem.9b01784 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50527569 (CHEMBL4440098) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate preincubated for 10 mins followed by NADPH addition and measured a... | J Med Chem 63: 3956-3975 (2020) Article DOI: 10.1021/acs.jmedchem.9b01784 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50527569 (CHEMBL4440098) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using testosterone as substrate preincubated for 10 mins followed by NADPH addition and measured after... | J Med Chem 63: 3956-3975 (2020) Article DOI: 10.1021/acs.jmedchem.9b01784 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50527569 (CHEMBL4440098) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of SGRG-K(Ac)-GG-K(Ac)-GLG-K(Ac)-GGA-K(Ac)-RHRKVGG-K-Biotin binding to N-terminal His6-tagged BRD4 bromodomain 2 (344 to 455 residues) (un... | J Med Chem 63: 3956-3975 (2020) Article DOI: 10.1021/acs.jmedchem.9b01784 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain-containing protein 4 (Homo sapiens (Human)) | BDBM50527569 (CHEMBL4440098) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Binding affinity to human partial length BRD4 bromodomain 1 long isoform (N44 to E168 residues) expressed in bacterial expression system by BROMOscan... | J Med Chem 63: 3956-3975 (2020) Article DOI: 10.1021/acs.jmedchem.9b01784 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bromodomain and extra-terminal motif (BET) (Homo sapiens (Human)) | BDBM50527569 (CHEMBL4440098) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Binding affinity to human partial length BRDT bromodomain 2 isoform b (K250 to E382 residues) expressed in bacterial expression system by bromoscan a... | J Med Chem 63: 3956-3975 (2020) Article DOI: 10.1021/acs.jmedchem.9b01784 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50527569 (CHEMBL4440098) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using tolbutamide as substrate preincubated for 10 mins followed by NADPH addition and measured after ... | J Med Chem 63: 3956-3975 (2020) Article DOI: 10.1021/acs.jmedchem.9b01784 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50527569 (CHEMBL4440098) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chinese Academy of Sciences Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using S-mephenytoin as substrate preincubated for 10 mins followed by NADPH addition and measured aft... | J Med Chem 63: 3956-3975 (2020) Article DOI: 10.1021/acs.jmedchem.9b01784 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||