

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50528204 CHEMBL4565968
SMILES: COc1cc(cc(OC)c1C(N)=O)-c1cncc(c1C)-c1ccc(cc1)N1CCNCC1
InChI Key: InChIKey=DDVZCQMFWBEKAW-UHFFFAOYSA-N
Data: 4 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528204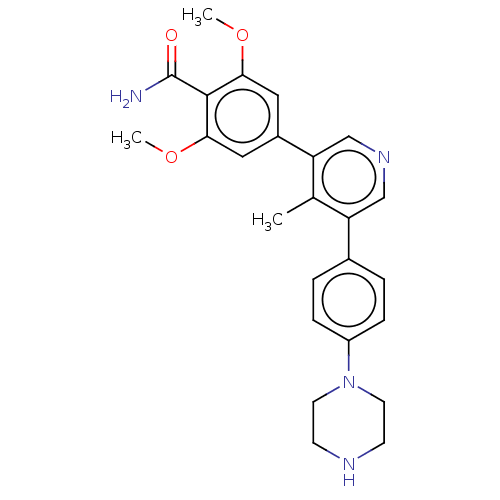 (CHEMBL4565968) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 178 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of C-terminal nanoluciferase-fused ALK2 (unknown origin) expressed in HEK293 cells incubated for 2 hrs followed by NanoBRET NanoGlo Substr... | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Activin receptor type-1 (Homo sapiens (Human)) | BDBM50528204 (CHEMBL4565968) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human ALK2 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50528204 (CHEMBL4565968) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 216 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of ALK5 (unknown origin) expressed in HEK293 cells transfected with CAGA-luciferase and Renilla luciferase reporter measured after 24 hrs ... | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50528204 (CHEMBL4565968) | PDB MMDB UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto Curated by ChEMBL | Assay Description Inhibition of human ALK5 using casein as substrate in presence of 10 uM [gamma33P] ATP by radioactive assay | J Med Chem 63: 4978-4996 (2020) Article DOI: 10.1021/acs.jmedchem.0c00395 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||