

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50529554 CHEMBL4567485
SMILES: COc1nc(Nc2cc(ccc2N[C@@H](c2cccc3OC(F)(F)Oc23)c2ncccc2Cl)S(C)(=O)=O)nc(OC)n1
InChI Key: InChIKey=ZKUCSIBYPOHTCH-IBGZPJMESA-N
Data: 1 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase, H3 lysine-79 specific (Homo sapiens (Human)) | BDBM50529554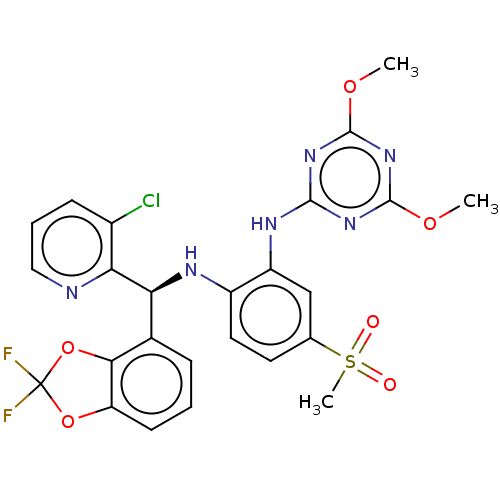 (CHEMBL4567485) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of 0.05 nM DOT1L (2 to 416 residues) (unknown origin) using biotinylated nucleosome as substrate and [3H]SAM as co-factor preincubated for... | ACS Med Chem Lett 10: 1655-1660 (2019) Article DOI: 10.1021/acsmedchemlett.9b00452 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||