

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50529979 CHEMBL4439229
SMILES: [#6]-[#6]-[#8]-[#6](=O)-[#7]-1-[#6]-[#6]\[#6](-[#6]-[#6]-1)=[#6]/C#Cc1cccc(c1)C#N
InChI Key: InChIKey=QWWMNIZBTUYTCH-UHFFFAOYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50529979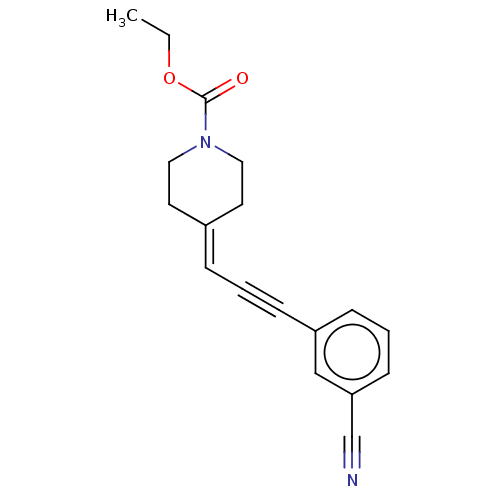 (CHEMBL4439229) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Recordati S.p.A. Curated by ChEMBL | Assay Description Displacement of [3H]MPEP from human mGlu5 receptor expressed in CHO-TREx cell membranes after 60 mins by liquid scintillation spectrometric analysis | J Med Chem 62: 1246-1273 (2019) Article DOI: 10.1021/acs.jmedchem.8b01226 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor 5 (Homo sapiens (Human)) | BDBM50529979 (CHEMBL4439229) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Recordati S.p.A. Curated by ChEMBL | Assay Description Displacement of [3H]MPEP from human mGlu5 receptor expressed in CHO-TREx cell membranes after 60 mins by liquid scintillation spectrometric analysis | J Med Chem 62: 1246-1273 (2019) Article DOI: 10.1021/acs.jmedchem.8b01226 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor (RAT) | BDBM50529979 (CHEMBL4439229) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Recordati S.p.A. Curated by ChEMBL | Assay Description Displacement of [3H]MPEP from rat mGlu1 receptor expressed in CHO-TREx cell membranes after 30 mins by liquid scintillation spectrometric analysis | J Med Chem 62: 1246-1273 (2019) Article DOI: 10.1021/acs.jmedchem.8b01226 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Metabotropic glutamate receptor (RAT) | BDBM50529979 (CHEMBL4439229) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Recordati S.p.A. Curated by ChEMBL | Assay Description Displacement of [3H]MPEP from rat mGlu1 receptor expressed in CHO-TREx cell membranes after 30 mins by liquid scintillation spectrometric analysis | J Med Chem 62: 1246-1273 (2019) Article DOI: 10.1021/acs.jmedchem.8b01226 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50529979 (CHEMBL4439229) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Recordati S.p.A. Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A2 using 3-cyano-7-ethoxycoumarin as substrate preincubated for 10 mins followed by substrate addition and measur... | J Med Chem 62: 1246-1273 (2019) Article DOI: 10.1021/acs.jmedchem.8b01226 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50529979 (CHEMBL4439229) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Recordati S.p.A. Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP2D6 using 3-[2-(N,N-diethylamino)ethyl]-7-methoxy-4-methylcoumarin as substrate preincubated for 10 mins followed ... | J Med Chem 62: 1246-1273 (2019) Article DOI: 10.1021/acs.jmedchem.8b01226 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50529979 (CHEMBL4439229) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Recordati S.p.A. Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP2D6 using 3-[2-(N,N-diethylamino)ethyl]-7-methoxy-4-methylcoumarin as substrate preincubated for 10 mins followed ... | J Med Chem 62: 1246-1273 (2019) Article DOI: 10.1021/acs.jmedchem.8b01226 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50529979 (CHEMBL4439229) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Recordati S.p.A. Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP3A4 using 7-benzyloxy-trifluoromethylcoumarin as substrate preincubated for 10 mins followed by substrate addition... | J Med Chem 62: 1246-1273 (2019) Article DOI: 10.1021/acs.jmedchem.8b01226 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50529979 (CHEMBL4439229) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Recordati S.p.A. Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP3A4 using 7-benzyloxy-trifluoromethylcoumarin as substrate preincubated for 10 mins followed by substrate addition... | J Med Chem 62: 1246-1273 (2019) Article DOI: 10.1021/acs.jmedchem.8b01226 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50529979 (CHEMBL4439229) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Recordati S.p.A. Curated by ChEMBL | Assay Description Inhibition of recombinant human CYP1A2 using 3-cyano-7-ethoxycoumarin as substrate preincubated for 10 mins followed by substrate addition and measur... | J Med Chem 62: 1246-1273 (2019) Article DOI: 10.1021/acs.jmedchem.8b01226 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||