

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50531921 CHEMBL4544914::US10676469, Compound 148
SMILES: CCc1cc(F)c2nc(N3CCC(CC3)N[C@@H]3CCCOC3)c(-c3nc(C)no3)c(C)c2c1
InChI Key: InChIKey=MLAUBNUNAKNFHB-LJQANCHMSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531921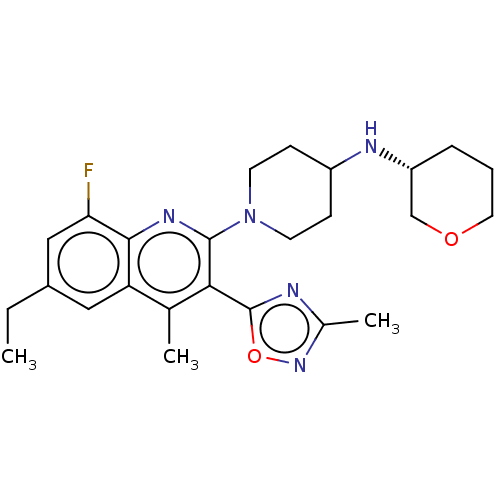 (CHEMBL4544914 | US10676469, Compound 148 | US11124...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
BLACKTHORN THERAPEUTICS, INC.; THE SCRIPPS RESEARCH INSTITUTE US Patent | Assay Description The cell line for the OPRK1 antagonist assay stably expresses the following elements: The carboxy terminus of the OPRK1 receptor has a 7-amino acid l... | US Patent US10676469 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50531921 (CHEMBL4544914 | US10676469, Compound 148 | US11124...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
BLACKTHORN THERAPEUTICS, INC.; THE SCRIPPS RESEARCH INSTITUTE US Patent | Assay Description The purpose of this assay is to confirm the potency and selectivity of compounds synthesized to be OPRK1 Antagonists. This assay monitors the OPRMu1 ... | US Patent US10676469 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531921 (CHEMBL4544914 | US10676469, Compound 148 | US11124...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused KOR (unknown origin) expressed in human U2OS cells co-expressing Tango-OPRK1-BLA assessed as inhibition of U-5... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50531921 (CHEMBL4544914 | US10676469, Compound 148 | US11124...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50531921 (CHEMBL4544914 | US10676469, Compound 148 | US11124...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused MOR (unknown origin) expressed in human U2OS cells assessed as inhibition of DAMGO-induced beta-arrestin migra... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50531921 (CHEMBL4544914 | US10676469, Compound 148 | US11124...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50531921 (CHEMBL4544914 | US10676469, Compound 148 | US11124...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description Antagonist activity at GAL4-VP16-fused DOR (unknown origin) expressed in human U2OS cells assessed as inhibition of DAMGO-induced beta-arrestin migra... | J Med Chem 62: 1761-1780 (2019) Article DOI: 10.1021/acs.jmedchem.8b01679 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||