

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50538008 CHEMBL4638732
SMILES: Cl.[H][C@]12C[C@@]1([H])N[C@H](COc1cncc(Cl)c1)C2
InChI Key: InChIKey=PVKSBMPVAXOXOX-MRALAVQDSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuronal acetylcholine receptor Alpha-4/Beta-2 (Homo sapiens (Human)) | BDBM50538008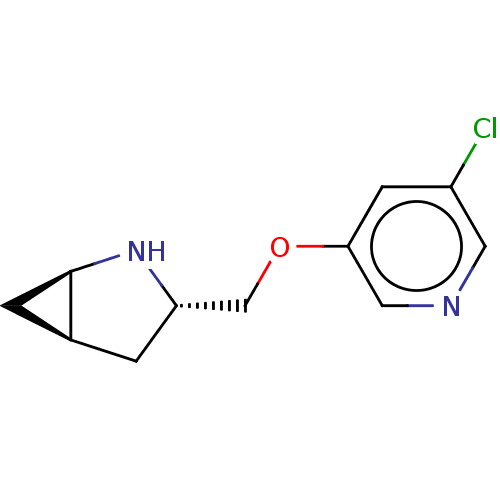 (CHEMBL4638732) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]cytisine from human alpha4beta2 nAChR expressed in CHOK1 cell membrane by microbeta scintillation counting method | J Med Chem 63: 2833-2853 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuronal acetylcholine receptor; alpha3/beta4 (Homo sapiens (Human)) | BDBM50538008 (CHEMBL4638732) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]epibatidine from human alpha3beta4 nAChR expressed in IMR32 cell membrane by microbeta scintillation counting method | J Med Chem 63: 2833-2853 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50538008 (CHEMBL4638732) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate incubated for 2 min in presence of NADPH by LC-MS/MS analysis | J Med Chem 63: 2833-2853 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50538008 (CHEMBL4638732) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | >4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Suven Life Sciences Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate incubated for 12 min in presence of NADPH by LC-MS/MS analysis | J Med Chem 63: 2833-2853 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||