

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50538514 CHEMBL4644274
SMILES: COc1ccc(cc1OC)-c1cnn2c(N)c(c(NC3CC(C)(C)N(C)C(C)(C)C3)nc12)-c1ccccc1
InChI Key: InChIKey=YRBWLEAMGAGYBK-UHFFFAOYSA-N
Data: 5 IC50
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kinesin-1 heavy chain/ Tyrosine-protein kinase receptor RET (Homo sapiens (Human)) | BDBM50538514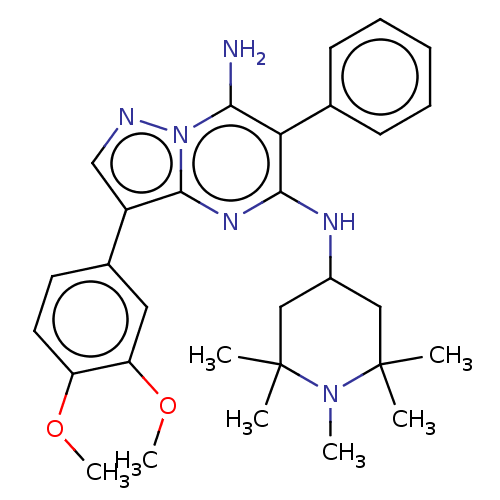 (CHEMBL4644274) | PDB KEGG UniProtKB/SwissProt UniProtKB/TrEMBL antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of KIF5B-RET (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction in cell viability incubated for 48 hrs by brightglo-l... | ACS Med Chem Lett 11: 558-565 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TEL/KDR (Homo sapiens (Human)) | BDBM50538514 (CHEMBL4644274) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of TEL/KDR (unknown origin) transfected in mouse Ba/F3 cells assessed as reduction in cell viability incubated for 48 hrs by brightglo-luc... | ACS Med Chem Lett 11: 558-565 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50538514 (CHEMBL4644274) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in human embryonic kidney cells by manual patch clamp assay | ACS Med Chem Lett 11: 558-565 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50538514 (CHEMBL4644274) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Displacement of [3H]-dofetilide from human ERG expressed in CHO cells incubated for 90 mins by microbeta scintillation counting method | ACS Med Chem Lett 11: 558-565 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coiled-coil domain-containing protein 6 (Homo sapiens (Human)) | BDBM50538514 (CHEMBL4644274) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
The Genomics Institute of the Novartis Research Foundation Curated by ChEMBL | Assay Description Inhibition of CCDC6/RET (unknown origin) transfected in human LC2/ad cells assessed as reduction in cell viability incubated for 6 days by CellTiter ... | ACS Med Chem Lett 11: 558-565 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||