

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
BDBM50538670 CHEMBL4642579
SMILES: Nc1ncnc2n(nc(Oc3cc(ccn3)C#N)c12)[C@H]1C[C@H](F)C1
InChI Key: InChIKey=USPIAMGRHIPYDO-MGCOHNPYSA-N
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein cereblon/Tyrosine-protein kinase SRC (Homo sapiens (Human)) | BDBM50538670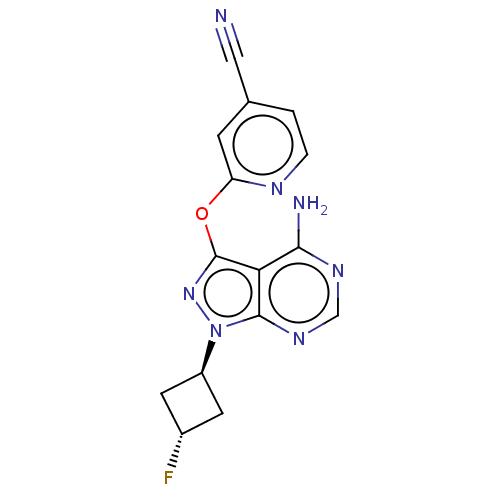 (CHEMBL4642579) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human Src expressed in Escherichia coli BL21 using Abltide peptide as substrate incubated for 20 to 40 mins by ELISA | J Med Chem 63: 6144-6163 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 1,3-beta-glucan synthase component GLS2 (Saccharomyces cerevisiae) | BDBM50538670 (CHEMBL4642579) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human ERG expressed in CHOK1 cells by patch-clamp electrophysiology assay | J Med Chem 63: 6144-6163 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50538670 (CHEMBL4642579) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 2.08E+4 | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of CYP1A2 in human liver microsomes using phenacetin as substrate measured after 10 mins by LC-MS/MS analysis | J Med Chem 63: 6144-6163 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50538670 (CHEMBL4642579) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of CYP3A4 in human liver microsomes using midazolam as substrate measured after 10 mins by LC-MS/MS analysis | J Med Chem 63: 6144-6163 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C19 (Homo sapiens (Human)) | BDBM50538670 (CHEMBL4642579) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of CYP2C19 in human liver microsomes using S-mephenytoin as substrate measured after 10 mins by LC-MS/MS analysis | J Med Chem 63: 6144-6163 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2D6 (Homo sapiens (Human)) | BDBM50538670 (CHEMBL4642579) | PDB UniProtKB/SwissProt UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of CYP2D6 in human liver microsomes using dextromethorphan as substrate measured after 10 mins by LC-MS/MS analysis | J Med Chem 63: 6144-6163 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50538670 (CHEMBL4642579) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Inhibition of CYP2C9 in human liver microsomes using diclofenac as substrate measured after 10 mins by LC-MS/MS analysis | J Med Chem 63: 6144-6163 (2020) | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||