

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: [H][C@@]1(CC[C@@]2([H])[C@]3([H])CC[C@]4([H])C[C@@H](CC[C@]4(C)[C@@]3([H])CC[C@]12C)OC(=O)[C@H](CC(O)=O)NC(=O)Cc1cc(=O)oc2cc(OC)ccc12)[C@H](C)CCC(=O)OCCOCCOCCOCCN=[N+]=[N-]
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Beta-galactoside alpha-2,6-sialyltransferase 1 (Homo sapiens (Human)) | BDBM50566003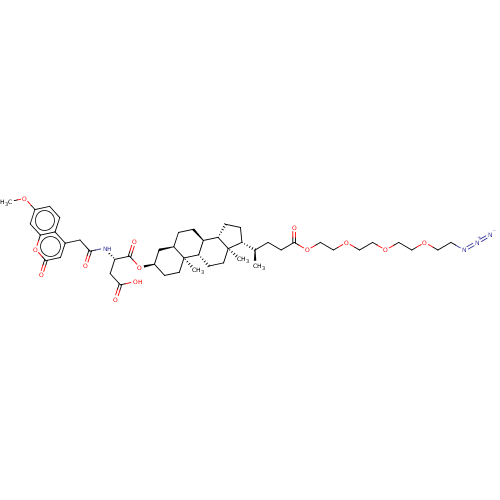 (CHEMBL4793980) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human alpha-2,6-ST6GAL1 assessed as reduction in sialylated-product formation using Gal-beta1-4GlcNac and CMP-NeuSAc incubated for 15 m... | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01477 BindingDB Entry DOI: 10.7270/Q2T43XVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||