

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: [H][C@@]1(O[C@H]2[C@H](O)[C@@H](O[C@]3([H])O[C@@H](C)[C@H](O)[C@@H](O)[C@H]3O)[C@]([H])(O[C@H]3CC[C@@]4(C)[C@@]([H])(CC[C@]5(C)[C@]4([H])CC=C4[C@]6([H])CC(C)(C)CC[C@@]6(CC[C@@]54C)C(=O)NCCc4ccccc4)C3(C)C)O[C@@H]2CO)O[C@@H](C)[C@H](O)[C@@H](O)[C@H]1O
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spike glycoprotein (SARS-CoV) | BDBM50567453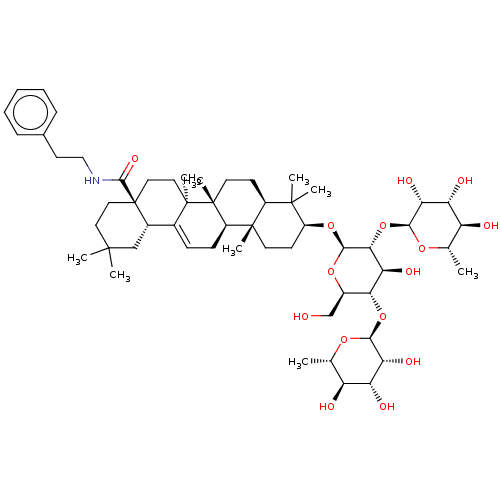 (CHEMBL4865746) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of spike glycoprotein S in SARS-CoV-2 pseudovirus infected human 293T/ACE2 cells assessed as inhibition of viral infection measured after ... | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113242 BindingDB Entry DOI: 10.7270/Q27W6GZC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||