Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569299
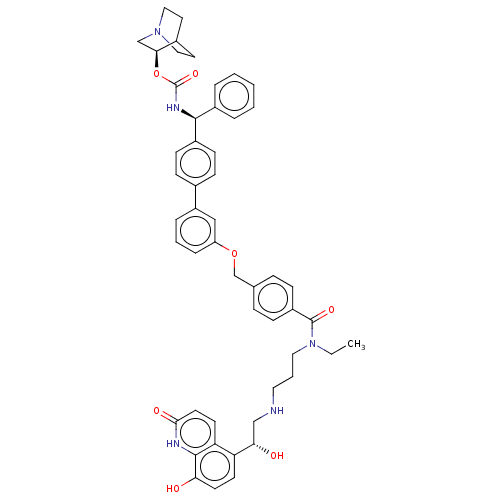
(CHEMBL4866806)Show SMILES CCN(CCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12)C(=O)c1ccc(COc2cccc(c2)-c2ccc(cc2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1 |r,wU:47.50,8.8,wD:42.46,(11.54,-25.97,;12.87,-25.19,;12.87,-23.65,;11.53,-22.89,;10.2,-23.66,;8.87,-22.89,;7.54,-23.67,;6.2,-22.9,;4.87,-23.68,;3.53,-22.91,;4.87,-25.22,;3.54,-25.99,;3.54,-27.53,;4.88,-28.3,;4.88,-29.84,;6.21,-27.53,;7.54,-28.3,;8.88,-27.53,;10.21,-28.3,;8.88,-25.98,;7.54,-25.2,;6.21,-25.98,;14.2,-22.88,;14.2,-21.34,;15.54,-23.64,;15.54,-25.18,;16.87,-25.94,;18.21,-25.17,;19.54,-25.94,;20.87,-25.16,;22.21,-25.92,;22.22,-27.46,;23.55,-28.22,;24.88,-27.45,;24.87,-25.9,;23.53,-25.14,;26.2,-25.12,;27.54,-25.88,;28.87,-25.11,;28.86,-23.56,;27.53,-22.8,;26.21,-23.58,;30.19,-22.78,;31.53,-23.54,;32.86,-22.76,;32.85,-21.22,;34.2,-23.52,;35.53,-22.75,;35.52,-21.22,;36.85,-20.44,;38.19,-21.21,;38.19,-22.75,;36.87,-23.52,;37.56,-22.17,;36.07,-21.77,;30.19,-21.24,;31.52,-20.47,;31.51,-18.93,;30.17,-18.17,;28.84,-18.95,;28.85,-20.49,;18.2,-23.62,;16.86,-22.86,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50569299

(CHEMBL4866806)Show SMILES CCN(CCCNC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12)C(=O)c1ccc(COc2cccc(c2)-c2ccc(cc2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1 |r,wU:47.50,8.8,wD:42.46,(11.54,-25.97,;12.87,-25.19,;12.87,-23.65,;11.53,-22.89,;10.2,-23.66,;8.87,-22.89,;7.54,-23.67,;6.2,-22.9,;4.87,-23.68,;3.53,-22.91,;4.87,-25.22,;3.54,-25.99,;3.54,-27.53,;4.88,-28.3,;4.88,-29.84,;6.21,-27.53,;7.54,-28.3,;8.88,-27.53,;10.21,-28.3,;8.88,-25.98,;7.54,-25.2,;6.21,-25.98,;14.2,-22.88,;14.2,-21.34,;15.54,-23.64,;15.54,-25.18,;16.87,-25.94,;18.21,-25.17,;19.54,-25.94,;20.87,-25.16,;22.21,-25.92,;22.22,-27.46,;23.55,-28.22,;24.88,-27.45,;24.87,-25.9,;23.53,-25.14,;26.2,-25.12,;27.54,-25.88,;28.87,-25.11,;28.86,-23.56,;27.53,-22.8,;26.21,-23.58,;30.19,-22.78,;31.53,-23.54,;32.86,-22.76,;32.85,-21.22,;34.2,-23.52,;35.53,-22.75,;35.52,-21.22,;36.85,-20.44,;38.19,-21.21,;38.19,-22.75,;36.87,-23.52,;37.56,-22.17,;36.07,-21.77,;30.19,-21.24,;31.52,-20.47,;31.51,-18.93,;30.17,-18.17,;28.84,-18.95,;28.85,-20.49,;18.2,-23.62,;16.86,-22.86,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

