Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50569300
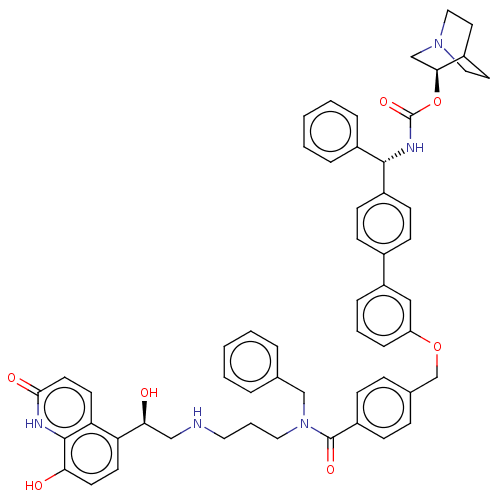
(CHEMBL4871702)Show SMILES O[C@@H](CNCCCN(Cc1ccccc1)C(=O)c1ccc(COc2cccc(c2)-c2ccc(cc2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:40.42,1.0,wD:35.38,(46.1,-20.06,;47.43,-20.83,;48.76,-20.05,;50.1,-20.82,;51.43,-20.05,;52.77,-20.81,;54.1,-20.04,;55.44,-20.81,;55.44,-22.35,;56.53,-23.43,;56.13,-24.91,;57.21,-25.99,;58.7,-25.59,;59.09,-24.1,;58,-23.03,;56.77,-20.03,;56.76,-18.49,;58.11,-20.8,;58.11,-22.33,;59.44,-23.1,;60.78,-22.33,;62.11,-23.09,;63.44,-22.31,;64.78,-23.08,;64.79,-24.61,;66.12,-25.38,;67.45,-24.6,;67.44,-23.05,;66.11,-22.3,;68.78,-22.27,;70.11,-23.04,;71.44,-22.26,;71.43,-20.71,;70.1,-19.96,;68.78,-20.73,;72.77,-19.93,;74.11,-20.69,;75.44,-19.91,;75.43,-18.37,;76.77,-20.68,;78.1,-19.9,;78.09,-18.37,;79.43,-17.6,;80.77,-18.36,;80.77,-19.9,;79.44,-20.68,;80.14,-19.32,;78.65,-18.92,;72.76,-18.39,;74.09,-17.62,;74.08,-16.08,;72.75,-15.32,;71.41,-16.1,;71.42,-17.64,;60.77,-20.78,;59.43,-20.02,;47.44,-22.37,;46.11,-23.14,;46.1,-24.69,;47.44,-25.46,;47.44,-27,;48.77,-24.68,;50.1,-25.45,;51.44,-24.68,;52.78,-25.46,;51.45,-23.14,;50.11,-22.36,;48.77,-23.13,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50569300

(CHEMBL4871702)Show SMILES O[C@@H](CNCCCN(Cc1ccccc1)C(=O)c1ccc(COc2cccc(c2)-c2ccc(cc2)[C@@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:40.42,1.0,wD:35.38,(46.1,-20.06,;47.43,-20.83,;48.76,-20.05,;50.1,-20.82,;51.43,-20.05,;52.77,-20.81,;54.1,-20.04,;55.44,-20.81,;55.44,-22.35,;56.53,-23.43,;56.13,-24.91,;57.21,-25.99,;58.7,-25.59,;59.09,-24.1,;58,-23.03,;56.77,-20.03,;56.76,-18.49,;58.11,-20.8,;58.11,-22.33,;59.44,-23.1,;60.78,-22.33,;62.11,-23.09,;63.44,-22.31,;64.78,-23.08,;64.79,-24.61,;66.12,-25.38,;67.45,-24.6,;67.44,-23.05,;66.11,-22.3,;68.78,-22.27,;70.11,-23.04,;71.44,-22.26,;71.43,-20.71,;70.1,-19.96,;68.78,-20.73,;72.77,-19.93,;74.11,-20.69,;75.44,-19.91,;75.43,-18.37,;76.77,-20.68,;78.1,-19.9,;78.09,-18.37,;79.43,-17.6,;80.77,-18.36,;80.77,-19.9,;79.44,-20.68,;80.14,-19.32,;78.65,-18.92,;72.76,-18.39,;74.09,-17.62,;74.08,-16.08,;72.75,-15.32,;71.41,-16.1,;71.42,-17.64,;60.77,-20.78,;59.43,-20.02,;47.44,-22.37,;46.11,-23.14,;46.1,-24.69,;47.44,-25.46,;47.44,-27,;48.77,-24.68,;50.1,-25.45,;51.44,-24.68,;52.78,-25.46,;51.45,-23.14,;50.11,-22.36,;48.77,-23.13,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

