

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: Clc1ccc2c(NCCCCCCNC3CCc4[nH]c(=O)ccc4C3)c3CCCCc3nc2c1
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carboxylic ester hydrolase (Rattus norvegicus (rat)) | BDBM50582082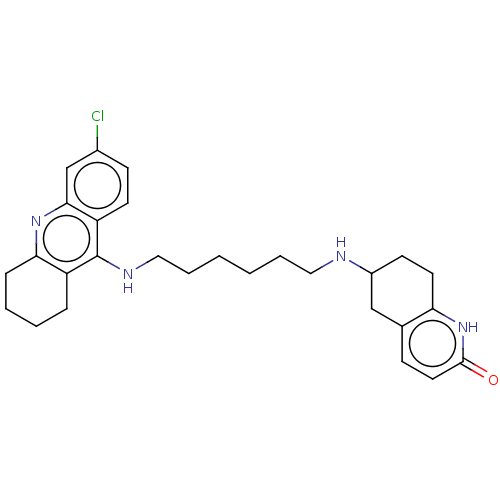 (CHEMBL5084050) | Reactome pathway KEGG UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 574 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat serum BChE using butyrylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50582082 (CHEMBL5084050) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of hERG expressed in CHO-K1 cells assessed as reduction in thallium influx incubated for 30 minutes by FLIPR | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Rattus norvegicus (rat)) | BDBM50582082 (CHEMBL5084050) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of rat cortex AChE using acetylthiocholine iodide as substrate by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2021.113827 BindingDB Entry DOI: 10.7270/Q2222ZP7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||