

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
null
SMILES: [H][C@]12CCN(C(C)=O)[C@@]1([H])CCN2[C@H]1CCC[C@H](C1)NC(=O)c1cc2c(F)ccc(C)c2[nH]1
InChI Key:
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Histone-lysine N-methyltransferase SETD2 (Homo sapiens (Human)) | BDBM50594410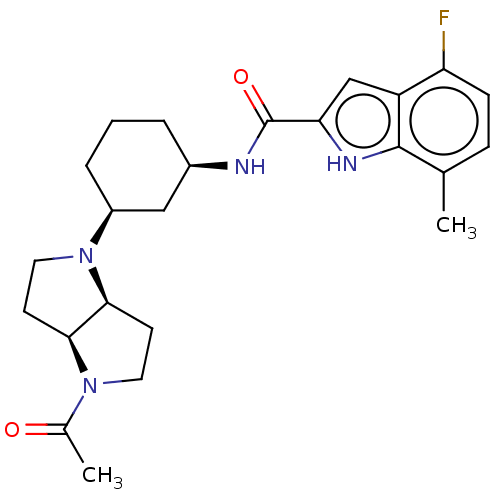 (CHEMBL5178972) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00167 BindingDB Entry DOI: 10.7270/Q2HQ43XC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone-lysine N-methyltransferase SETD2 (Homo sapiens (Human)) | BDBM50594410 (CHEMBL5178972) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1021/acsmedchemlett.2c00167 BindingDB Entry DOI: 10.7270/Q2HQ43XC | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||