 Found 4 hits for monomerid = 50595405
Found 4 hits for monomerid = 50595405 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50595405
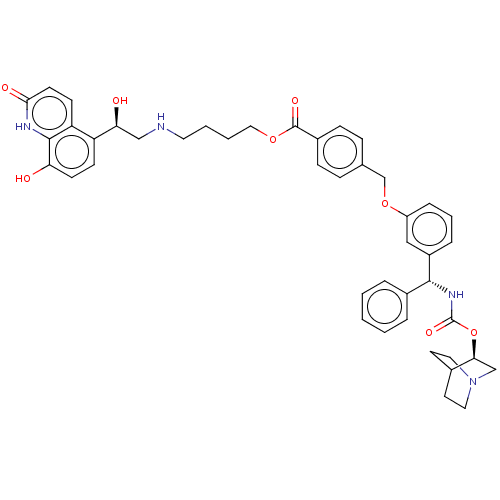
(CHEMBL5184598)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,23.24,wD:28.28,(-14.67,-1.16,;-13.33,-1.93,;-12,-1.16,;-10.67,-1.93,;-9.33,-1.16,;-8,-1.93,;-6.66,-1.16,;-5.33,-1.93,;-4,-1.16,;-2.66,-1.93,;-2.66,-3.47,;-1.33,-1.16,;.01,-1.93,;1.34,-1.16,;1.34,.38,;2.67,1.15,;4.01,.38,;5.34,1.15,;6.67,.39,;8,1.16,;8,2.69,;6.67,3.46,;5.34,2.69,;6.67,5,;8,5.77,;9.34,5,;9.34,3.46,;10.67,5.77,;12.01,5,;12.01,3.46,;13.34,2.69,;12.63,3.93,;14.05,4.54,;13.34,5.77,;14.67,5,;14.67,3.46,;5.34,5.77,;5.34,7.31,;4,8.08,;2.67,7.31,;2.67,5.77,;4,5,;-0,1.15,;-1.33,.38,;-13.33,-3.47,;-14.67,-4.24,;-14.67,-5.78,;-13.34,-6.54,;-13.34,-8.08,;-12,-5.77,;-10.67,-6.54,;-9.34,-5.77,;-8.01,-6.54,;-9.34,-4.24,;-10.67,-3.47,;-12,-4.24,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50595405

(CHEMBL5184598)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,23.24,wD:28.28,(-14.67,-1.16,;-13.33,-1.93,;-12,-1.16,;-10.67,-1.93,;-9.33,-1.16,;-8,-1.93,;-6.66,-1.16,;-5.33,-1.93,;-4,-1.16,;-2.66,-1.93,;-2.66,-3.47,;-1.33,-1.16,;.01,-1.93,;1.34,-1.16,;1.34,.38,;2.67,1.15,;4.01,.38,;5.34,1.15,;6.67,.39,;8,1.16,;8,2.69,;6.67,3.46,;5.34,2.69,;6.67,5,;8,5.77,;9.34,5,;9.34,3.46,;10.67,5.77,;12.01,5,;12.01,3.46,;13.34,2.69,;12.63,3.93,;14.05,4.54,;13.34,5.77,;14.67,5,;14.67,3.46,;5.34,5.77,;5.34,7.31,;4,8.08,;2.67,7.31,;2.67,5.77,;4,5,;-0,1.15,;-1.33,.38,;-13.33,-3.47,;-14.67,-4.24,;-14.67,-5.78,;-13.34,-6.54,;-13.34,-8.08,;-12,-5.77,;-10.67,-6.54,;-9.34,-5.77,;-8.01,-6.54,;-9.34,-4.24,;-10.67,-3.47,;-12,-4.24,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(GUINEA PIG) | BDBM50595405

(CHEMBL5184598)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,23.24,wD:28.28,(-14.67,-1.16,;-13.33,-1.93,;-12,-1.16,;-10.67,-1.93,;-9.33,-1.16,;-8,-1.93,;-6.66,-1.16,;-5.33,-1.93,;-4,-1.16,;-2.66,-1.93,;-2.66,-3.47,;-1.33,-1.16,;.01,-1.93,;1.34,-1.16,;1.34,.38,;2.67,1.15,;4.01,.38,;5.34,1.15,;6.67,.39,;8,1.16,;8,2.69,;6.67,3.46,;5.34,2.69,;6.67,5,;8,5.77,;9.34,5,;9.34,3.46,;10.67,5.77,;12.01,5,;12.01,3.46,;13.34,2.69,;12.63,3.93,;14.05,4.54,;13.34,5.77,;14.67,5,;14.67,3.46,;5.34,5.77,;5.34,7.31,;4,8.08,;2.67,7.31,;2.67,5.77,;4,5,;-0,1.15,;-1.33,.38,;-13.33,-3.47,;-14.67,-4.24,;-14.67,-5.78,;-13.34,-6.54,;-13.34,-8.08,;-12,-5.77,;-10.67,-6.54,;-9.34,-5.77,;-8.01,-6.54,;-9.34,-4.24,;-10.67,-3.47,;-12,-4.24,)| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor
(Cavia porcellus) | BDBM50595405

(CHEMBL5184598)Show SMILES O[C@@H](CNCCCCOC(=O)c1ccc(COc2cccc(c2)[C@H](NC(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1)c1ccc(O)c2[nH]c(=O)ccc12 |r,wU:1.0,23.24,wD:28.28,(-14.67,-1.16,;-13.33,-1.93,;-12,-1.16,;-10.67,-1.93,;-9.33,-1.16,;-8,-1.93,;-6.66,-1.16,;-5.33,-1.93,;-4,-1.16,;-2.66,-1.93,;-2.66,-3.47,;-1.33,-1.16,;.01,-1.93,;1.34,-1.16,;1.34,.38,;2.67,1.15,;4.01,.38,;5.34,1.15,;6.67,.39,;8,1.16,;8,2.69,;6.67,3.46,;5.34,2.69,;6.67,5,;8,5.77,;9.34,5,;9.34,3.46,;10.67,5.77,;12.01,5,;12.01,3.46,;13.34,2.69,;12.63,3.93,;14.05,4.54,;13.34,5.77,;14.67,5,;14.67,3.46,;5.34,5.77,;5.34,7.31,;4,8.08,;2.67,7.31,;2.67,5.77,;4,5,;-0,1.15,;-1.33,.38,;-13.33,-3.47,;-14.67,-4.24,;-14.67,-5.78,;-13.34,-6.54,;-13.34,-8.08,;-12,-5.77,;-10.67,-6.54,;-9.34,-5.77,;-8.01,-6.54,;-9.34,-4.24,;-10.67,-3.47,;-12,-4.24,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Maryland
Curated by ChEMBL
| |
J Med Chem 48: 4500-3 (2005)
Article DOI: 10.1021/acs.jmedchem.2c00609
BindingDB Entry DOI: 10.7270/Q2TH8RQS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data